Clinical analysis of 54 cases of lung cancer treated by domestic carbon ion system
Clinical analysis of 54 cases of lung cancer treated by domestic carbon ion system
中华放射肿瘤学杂志 2024 年4 月第 33 卷第 4 期 Chin J Radiat Oncol, April 2024, Vol. 33, No. 4
Pan Xin1, Zhang Yihe1, Ma Tong1, Wang Xin1, Yang Yuling1, Qin Tianyan2, Lyu Caixia2, Li Pengqing2, Ye Yancheng1, Zhang Yanshan1
1Department of Radiation Oncology, Heavy ion Center, Wuwei Cancer Hospital of Gansu Province, Wuwei 733000, China; 2Registration / Follow ‐ up Center, Heavy ion Center, Wuwei Cancer Hospital of Gansu Province, Wuwei 733000, China
Corresponding author: Zhang Yanshan, Email: 13830510999@163.com
【Abstract】 Objective To evaluate clinical prognosis and prognostic factors of patients with early stage (Ⅰ stage) and locally advanced (Ⅱ/Ⅲ stage) lung cancer treated with carbon ion radiotherapy (CIRT). Methods Clinical data, treatment, adverse reactions, survival and so on of 54 lung cancer patients who received CIRT and follow ‐ up in the Heavy Ion Center of Wuwei Cancer Hospital of Gansu Province from March 2020 to September 2022 were retrospectively analyzed. The survival curve was plotted using Kaplan‐Meier method. Difference tests were performed using log‐rank test. Logistic regression analysis was used to identify prognostic factors. Results According to inclusion and exclusion criteria, 54 patients were enrolled in the study, including 10 patients with early stage lung cancer and 44 patients with locally advanced lung cancer. The median follow ‐ up time for 10 patients with early stage lung cancer was 11.0 (6.75, 17.25) months, and the median dose of irradiation was 60 Gy [relative biological effect (RBE)]. Upon the last follow ‐up, 3 patients had complete response (CR) and 3 patients had partial response (PR). Four patients had stable disease (SD) and no progressive disease (PD). The 1 ‐ year and 2 ‐ year local control rates (LCR), progression‐free survival (PFS) rates and overall survival (OS) rates were 100%. During treatment and follow ‐ up, 2 patients developed grade 1 radiation pneumonia, 1 case of grade 2 radiation pneumonia, 1 case of chest wall injury (chest wall pain), and there were no adverse reactions greater than grade 2. The median follow‐up time of 44 patients with locally advanced stage was 12.5 (4.25, 21.75) months, and the median irradiation dose was 72 Gy (RBE). Thirty ‐ two (73%) patients received concurrent chemotherapy during treatment, 20 (45%) patients received sequential chemotherapy after treatment, 14 (32%) patients received immune maintenance therapy and 3 (7%) patients obtained PD and received targeted drugs. Upon the last follow‐up, 3 (7%) patients had CR, 17 (39%) patients had PR, 19 (43%) patients obtained SD, and 5 (11%) patients had PD. The 1‐year and 2‐year LCR were 96.0% and 87.3%, 90.9% and 84.1% for the 1‐year and 2‐year PFS rates, and 93.2% and 86.4% for the 1‐year and 2‐year OS rates, respectively. The median OS and PFS of patients were not reached. Multivariate logistic regression analysis showed that maintenance therapy after radiotherapy (P=0.027) and clinical target volume (CTV) irradiation volume (P=0.028) were the factors affecting PFS. Simultaneous chemoradiotherapy (P=0.042) and maintenance therapy after radiotherapy (P=0.020) were the factors affecting OS. And gross tumor volume (GTV) ≥215 ml (P=0.068) might be an independent risk factor for grade 2 and above radiation pneumonia. Conclusions The domestic carbon ion system has definite clinical effect and controllable toxic and side effects in the treatment of early stage and locally advanced lung cancer. The combination of synchronous chemotherapy and further maintenance treatment can significantly improve clinical prognosis of patients without significantly increasing the risk of toxic and side effects.
【Key words】 Radiotherapy; Carbon ion; Lung neoplasms, early stage; Lung neoplasms, locally advanced; Adverse reactions; Treatment outcome
Fund programs: Key Science and Technology R&D Plan of Gansu Province ‐ Construction of Heavy Ion Treatment Center for Social Development (19YF3FH001); Natural Science Foundation of Gansu Province ‐ 2021 Innovation Base and Talents Program of Science and Technology Department of Gansu Province (21JR7RH896)
Lung cancer is one of the most common malignancies, with approximately 350 deaths per day in the United States, making it the second most prevalent cancer and the leading cause of cancer-related mortality in both men and women [1]. Chinese statistics show that about 631,000 people die from lung cancer annually in China [2]. While lung cancer incidence rates are declining in some Western countries, they continue to rise in China. Carbon ion therapy, also known as carbon ion radiotherapy, is a form of heavy ion radiotherapy classified as particle therapy. Conventional photon radiotherapy differs fundamentally from particle beams (such as protons and carbon ions) in their physical properties. In current clinical practice, carbon ions are the most commonly used heavy ions, often interchangeably referred to as heavy ions in daily communication. Globally, there are currently 67 operational particle therapy centers, including 62 proton centers, 5 carbon ion centers, and 6 centers offering both proton and carbon ion therapy (data from the Particle Therapy Co-Operative Group website www.ptcog.ch). The National Institute of Radiological Sciences (NIRS) in Japan is one of the pioneering centers with the most extensive experience in carbon ion therapy, having clinically treated lung cancer patients with carbon ions for nearly 30 years. In 1994, NIRS initiated dose-escalation studies for peripheral early-stage non-small cell lung cancer (NSCLC) (18 fractions/6 weeks). Between 1994-1999, NIRS conducted phase I-II dose-escalation clinical trials for stage I peripheral NSCLC, ultimately establishing the optimal dose and confirming the feasibility of hypofractionated carbon ion radiotherapy [3]. From 1995-2015, 141 locally advanced NSCLC patients were treated: the 2-year local control rate (LCR), progression-free survival (PFS) rate, and overall survival (OS) rate were 80.3%, 40.2%, and 58.7% respectively. Regarding adverse effects, there was 1 case (0.7%) of grade 4 (mediastinal hemorrhage), 5 cases (3.5%) of grade 3 (radiation pneumonitis), and 1 case (0.7%) of grade 3 (bronchial fistula), demonstrating both the efficacy and acceptable toxicity profile of carbon ion therapy for locally advanced NSCLC. Particularly for elderly patients or those with comorbidities who cannot undergo surgery or conventional chemoradiotherapy, carbon ion therapy has shown comparable safety and efficacy [4]. In March 2020, China's first domestically developed heavy (carbon) ion cancer treatment system was officially put into clinical use at the Heavy Ion Center of Wuwei Cancer Hospital in Gansu Province. This study retrospectively analyzes the clinical treatment and follow-up data of 54 lung cancer patients treated with carbon ions at our center.
Materials and Methods
1. General information: We retrospectively analyzed clinical data of 54 lung cancer patients who completed carbon ion therapy and follow-up at the Heavy Ion Center of Wuwei Cancer Hospital from March 2020 to September 2022. Inclusion criteria: histologically confirmed NSCLC patients staged using brain MRI, chest CT, and positron emission tomography-computed tomography (PET-CT) (AJCC 8th edition); Karnofsky Performance Status (KPS) 70-100; measurable solid tumors; patients unwilling, unsuitable, or refusing surgery; without other progressive malignancies. Exclusion criteria: suspected invasion of trachea, major blood vessels, heart or carina; incomplete follow-up data. This study was approved by the Ethics Committee of Wuwei Cancer Hospital (Approval No.: 2021-Ethical Review-05).
2. Carbon ion therapy
(1) Pre-treatment positioning: Patients were immobilized using thermoplastic chest masks and vacuum cushions. CT scans were performed in supine or prone position depending on tumor location, with 3mm slice thickness and spacing, scanning from the cricothyroid membrane to the lower border of L1 vertebra, including 4D-CT for respiratory motion assessment.
(2) Target delineation: Standardized target delineation was performed (with MR or PET-CT fusion when necessary). Gross tumor volume (GTV): radiologically visible tumor (referencing contrast-enhanced CT, MRI or PET-CT). GTVnd represented metastatic mediastinal lymph nodes, defined as nodes with short-axis diameter ≥1cm and/or PET-CT positive and/or endobronchial ultrasound positive. For early-stage (stage I) lung cancer: Clinical target volume (CTV) was GTV plus 0.5cm margin, internal target volume (ITV) encompassed CTV motion observed on 4D-CT, and planning target volume (PTV) was ITV plus 3-5mm margin with appropriate adjustments near organs at risk (OARs). Prescription dose was 60-76Gy [relative biological effectiveness (RBE)] in 3-12 fractions. For locally advanced lung cancer (stage IIA-IIB, IIIA-IIIC): Elective nodal irradiation (ENI) was used. CTV included GTV + GTVnd with 0.5cm margin plus prophylactic lymph node regions (based on risk of nodal involvement, typically covering stations II, IV, V, VII), with OAR adjustments. ITV represented CTV motion on 4D-CT. PTV1 was ITV plus 3-5mm margin, prescribed 48Gy (RBE) in 12-16 fractions. PTV2 was GTV delineated on 4D-CT plus 0.5cm margin for primary tumor boost, prescribed 20-28Gy (RBE) in 4-7 fractions. Treatment sequence was PTV1 followed by PTV2, achieving 48Gy (RBE) to involved nodes and prophylactic regions, and 68-76Gy (RBE) total dose to primary tumor. OAR dose constraints followed NIRS guidelines: main bronchus Dmax<60Gy (RBE), esophagus Dmax<50Gy (RBE), spinal cord Dmax<30Gy (RBE), using horizontal and vertical carbon ion beams.
3. Systemic comprehensive treatment: All patients underwent multidisciplinary team (MDT) evaluation. Treatment followed the latest National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines for systemic therapy recommendations, including concurrent chemotherapy regimens and post-radiotherapy maintenance therapy tailored to individual conditions.
4. Efficacy evaluation and toxicity assessment: Follow-up began on day 1 post-radiotherapy, with CT scans monthly for first 2 months, then every 3 months until September 30, 2022. Acute toxicities were graded using CTCAE v5.0, while late toxicities used RTOG criteria. Tumor response was assessed per RECIST 1.1. LCR=(CR+PR+SD)/evaluable cases×100%; PFS was defined from radiotherapy start to disease progression or any-cause death; OS was from radiotherapy start to any-cause death or last follow-up.
5. Statistical analysis: SPSS 22.0 was used for analysis. Normally distributed data were presented as mean±SD, non-normal data as median (Q1, Q3), and percentages for categorical variables. T-tests and chi-square tests analyzed count data. Survival analysis used Kaplan-Meier method with log-rank tests. Multivariate logistic regression analyzed clinical factors associated with adverse events. P<0.05 was considered statistically significant.
Results
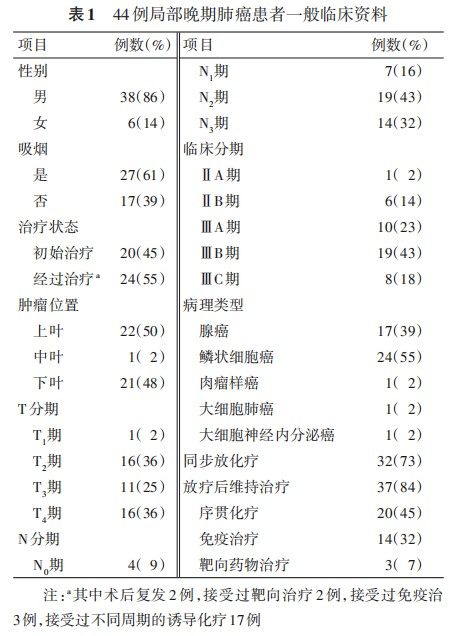
1. Baseline characteristics: 54 patients were enrolled (10 early-stage, 44 locally advanced). Early-stage patients: median age 59.5 (53.5, 66.7) years, 4 males/6 females; 9 stage IA, 1 stage IB; T1a/T1b/T1c/T2a: 2/3/4/1 cases; 9 initial treatments, 1 local recurrence after radiotherapy; 7 adenocarcinomas, 3 squamous cell carcinomas; 2 smokers; 4 upper lobe, 6 lower lobe tumors; median dose 60 (60, 68.8)Gy (RBE) in 3-12 fractions; median CTV volume 36.2 (32.25, 83.10)ml.
Locally advanced patients: median age 63 (56.3, 67.0) years; 32 (73%) received concurrent chemotherapy. Post-radiotherapy, 20 (45%) received sequential chemotherapy, 14 (32%) immunotherapy maintenance, and 3 (7%) targeted therapy after progression. Primary tumor median GTV volume was 215.4 (122.1, 399.9)ml with median dose 72 (65.0, 76.0)Gy (RBE). With ENI, median CTV volume for involved nodes and prophylactic regions was 425.4 (290.3, 555.2)ml receiving 48Gy (RBE) [5-7]. Other baseline data are shown in Table 1.
2. Analysis of adverse reactions and influencing factors: All patients completed the carbon ion treatment plan as scheduled without interruption due to severe adverse reactions. Among the 10 early-stage lung cancer patients who received irradiation of only the primary lesion, there were 2 cases of grade 1 radiation pneumonitis, 1 case of grade 2 radiation pneumonitis, and 1 case of chest wall injury (chest wall pain), with no adverse reactions exceeding grade 2.
For the 44 locally advanced lung cancer patients treated with elective nodal irradiation (covering both primary lesions and prophylactic mediastinal lymph node regions), adverse reactions included:
• 2 cases (5%) of grade 1 skin reactions
• 9 cases (20%) of radiation esophagitis (6 grade 1 and 3 grade 2)
• 14 cases (32%) of varying degrees of radiation pneumonitis (7 grade 1, 5 grade 2, and 2 grade 3)
• 1 case (2%) of chest wall injury
No grade 4 adverse reactions were observed.
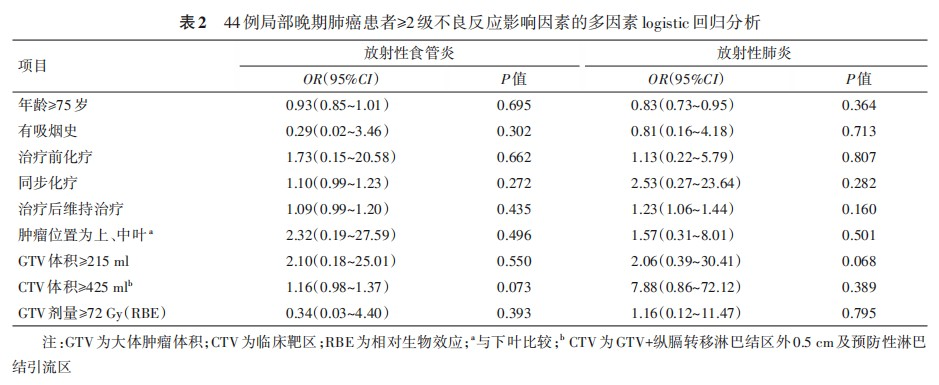
Stepwise multivariate logistic regression analysis of risk factors for ≥grade 2 adverse reactions in locally advanced lung cancer patients did not identify any statistically significant variables (P<0.05). When relaxing the P-value threshold to 0.1, GTV≥215ml (OR=2.06, 95%CI: 0.39-30.41, P=0.068) might represent an independent risk factor for developing ≥grade 2 radiation pneumonitis (Table 2).
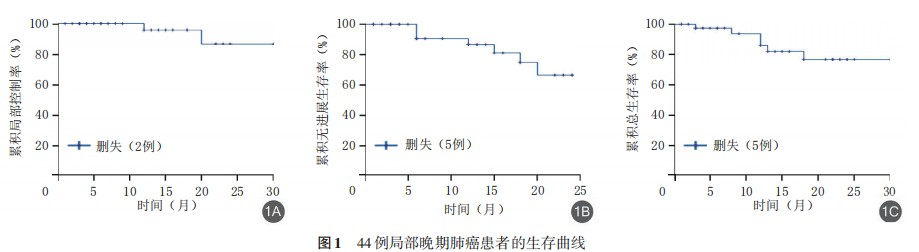
3. Analysis of patient prognosis and influencing factors: The median follow-up time for the 10 early-stage lung cancer patients was 11.0 (6.75, 17.25) months. At the last follow-up, among these 10 patients: 3 achieved complete response (CR), 3 showed partial response (PR), and 4 had stable disease (SD), with no cases of disease progression. The 1-year and 2-year local control rate (LCR), progression-free survival (PFS) rate, and overall survival (OS) rate were all 100%.
For the 44 locally advanced patients, the median follow-up time was 12.5 (4.25, 21.75) months. At the last follow-up:
• 3 cases (7%) achieved CR
• 17 cases (39%) showed PR
• 19 cases (43%) had SD
• 5 cases (11%) experienced disease progression
The survival rates were:
• 1-year and 2-year LCR: 96.0% (95%CI: 88.4%-103.6%) and 87.3% (95%CI: 69.5%-105.1%) respectively
• 1-year and 2-year PFS rates: 90.9% (95%CI: 74.4%-99.0%) and 84.1% (95%CI: 44.2%-89.2%) respectively
• 1-year and 2-year OS rates: 93.2% (95%CI: 73.9%-98.9%) and 86.4% (95%CI: 60.8%-93.8%) respectively
The median OS and PFS had not yet been reached (Figure 1).
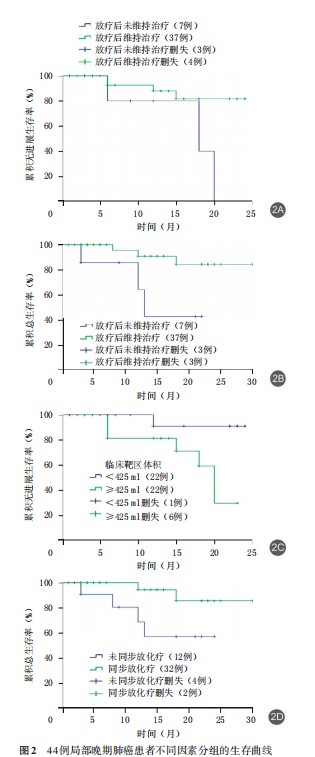
Multivariate analysis of 44 locally advanced lung cancer patients was performed to identify potential prognostic factors for PFS and OS across different subgroups. The results demonstrated:
1. For PFS:
• Post-radiotherapy maintenance therapy (P=0.027)
• CTV volume (P=0.028)
were identified as significant influencing factors.
2. For OS:
• Concurrent chemoradiotherapy (P=0.042)
• Post-radiotherapy maintenance therapy (P=0.020)
were identified as significant influencing factors.
Patients receiving post-radiotherapy maintenance therapy showed:
• Longer PFS: 21.55 months (95%CI: 19.33-23.76) vs 16.40 months (95%CI: 10.66-22.15) for non-maintenance group
• Longer OS: 27.40 months (95%CI: 24.66-30.14) vs 15.21 months (95%CI: 9.77-20.66) for non-maintenance group
(Figure 2A, 2B)
Regarding CTV volume:
• Patients with CTV <425ml had longer PFS (22.91 months, 95%CI: 20.87-24.95)
• Compared to those with CTV ≥425ml (17.52 months, 95%CI: 14.26-20.78)
(Figure 2C)
For treatment modality:
• Patients receiving concurrent chemoradiotherapy showed longer OS (27.97 months, 95%CI: 25.34-30.60)
• Compared to those without concurrent chemotherapy (17.82 months, 95%CI: 13.02-22.62)
(Figure 2D)
Discussion
Charged particle therapy is widely recognized as a cutting-edge technology in oncology. Although more effective than X-rays, proton therapy remains the most commonly used modality in the United States, Europe, and Asia, with only a few centers employing heavy ions. Since 1994, Japan's National Institute of Radiological Sciences (NIRS) has been utilizing high-energy carbon ions for tumor treatment [8]. As of 2022, over 46,800 patients worldwide have undergone heavy ion therapy (data from the Particle Therapy Co-Operative Group website).
In the phase I-II prospective carbon ion radiotherapy study by Miyamoto et al. [9], patients with stage I NSCLC (T1 tumors) received a fixed dose of 52.8 Gy (RBE), while T2 tumors received 60 Gy (RBE), administered four times weekly, achieving an overall 5-year local control rate (LCR) of 90%. Saitoh et al. [10] conducted a phase II prospective study (GUNMA0701) on hypofractionated carbon ion therapy for peripheral stage I NSCLC, treating 37 patients. The 2- and 5-year LCR were 91.2% and 88.1%, respectively, with 2- and 5-year overall survival (OS) rates of 91.9% and 74.9%. Two patients with pre-existing lung disease experienced ≥grade 2 pulmonary toxicity, while one patient without lung disease had <grade 2 toxicity. A multicenter prospective study of stereotactic body radiotherapy (SBRT) for T1N0M0 NSCLC reported 3-year OS rates of 76.5% and 59.9% for operable and inoperable patients, respectively, though with a higher incidence of severe pulmonary toxicity [11]. The RTOG 0236 trial in the U.S. employed a photon regimen of 60 Gy in 3 fractions, resulting in a 16% rate of grade 3-4 pneumonitis [12].
In our study, all 10 early-stage NSCLC patients received hypofractionated carbon ion therapy (50–76 Gy (RBE) in 3–12 fractions, tailored to tumor proximity to organs at risk). Two patients developed grade 1 radiation pneumonitis, one had grade 2 pneumonitis, and one experienced chest wall pain, with no other adverse events. At the last follow-up, none exhibited local recurrence or distant metastasis, with 1- and 2-year LCR, PFS, and OS rates all at 100%.
Locally advanced NSCLC is typically treated with surgery or chemoradiotherapy. In the RTOG 0617 trial by Bradley et al. [13], photon-based chemoradiotherapy (60 Gy) yielded median PFS and OS of 11.8 and 28.7 months, respectively, with 2-year PFS and OS rates of 30.7% and 57.6%. Yamamoto et al. [14] reported median PFS and OS of 9.5 and 22.0 months in a phase III trial of cisplatin/paclitaxel chemoradiotherapy. Takahashi et al. [5] and Karube et al. [15] treated 62 and 64 stage II-III NSCLC patients with carbon ions, achieving 2-year PFS and OS rates of 42.3% and 51.9–62.2%, respectively. Hayashi et al. [4] analyzed 144 patients treated with carbon ions alone, reporting median PFS and OS of 11.6 and 29.3 months, and 2-year rates of 40.2% and 58.7%.
Our cohort of 44 locally advanced patients demonstrated superior outcomes: median PFS and OS were not reached, with 1- and 2-year PFS rates of 90.9% and 84.1%, and OS rates of 93.2% and 86.4%. This improvement may stem from adherence to CSCO/NCCN guidelines, including concurrent chemotherapy (73%), sequential chemotherapy (45%), immunotherapy maintenance (32%), and targeted therapy for progression (7%). Multivariate analysis identified post-radiotherapy maintenance (P=0.027) and CTV volume (P=0.028) as PFS predictors, and concurrent chemoradiotherapy (P=0.042) and maintenance therapy (P=0.020) as OS predictors. Patients with CTV <425 ml had longer PFS (22.91 vs. 17.52 months), and those receiving concurrent chemotherapy had superior OS (27.97 vs. 17.82 months). These findings underscore the prognostic benefits of combining carbon ions with systemic therapies.
Toxicity profiles were favorable: no grade >3 events occurred in early-stage patients, while locally advanced patients exhibited grade 1–2 esophagitis (20%) and pneumonitis (32%, including 4.5% grade 3). Compared to photon studies reporting 7–20% grade ≥3 toxicities [13–14,16], our results align with Hayashi et al. [4] (3.5% grade 2 esophagitis, 10.6% grade ≥2 pneumonitis), confirming carbon ions' safety even with elective nodal irradiation and combined therapies. GTV ≥215 ml (OR=2.06, P=0.068) emerged as a potential risk factor for grade ≥2 pneumonitis, while other factors (e.g., chemotherapy, dose escalation) showed no significant impact.
Limitations
This first report of China’s domestic carbon ion system has limitations: (1) single-center retrospective design; (2) limited follow-up duration; (3) challenges in conducting phase III trials due to cost and patient preferences. However, with national expansion of heavy ion centers, future high-level studies are anticipated. Current data affirm carbon ions’ efficacy and manageable toxicity for early and locally advanced NSCLC, particularly when integrated with modern systemic therapies.
Conflicts of Interest
All authors declare no conflicts of interest.
Authors’ Contributions
Pan Xin and Zhang Yanshan: Study design, implementation, and manuscript drafting; Zhang Yihe and Ye Yancheng: Data collection, conceptualization, and critical revision; Qin Tianyan and Ma Tong: Literature review and data analysis; others participated in research.
References
[1] Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022[J]. CA Cancer J Clin, 2022,72(1):7-33. DOI: 10.3322/caac.21708.
[2] Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015[J]. Chinese Journal of Oncology, 2019, 41(1): 19-28. DOI: 10.3760/cma.j.issn.0253-3766.2019.01.005.
Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015[J]. Chin J Oncol,2019,41(1):19-28. DOI: 10.3760/cma.j.issn.0253-3766.2019.01.005.
[3] Miyamoto T, Yamamoto N, Nishimura H, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer[J]. Radiother Oncol, 2003, 66(2): 127-140. DOI: 10.1016/s0167-8140(02)00367-5.
[4] Hayashi K, Yamamoto N, Nakajima M, et al. Clinical outcomes of carbon-ion radiotherapy for locally advanced non-small-cell lung cancer[J]. Cancer Sci, 2019, 110(2):734-741. DOI: 10.1111/cas.13890.
[5] Takahashi W, Nakajima M, Yamamoto N, et al. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC) [J]. Cancer, 2015,121(8):1321-1327. DOI: 10.1002/cncr.29195.
[6] Hayashi K, Yamamoto N, Karube M, et al. Prognostic analysis of radiation pneumonitis: carbon-ion radiotherapy in patients with locally advanced lung cancer[J]. Radiat Oncol, 2017, 12(1): 91. DOI: 10.1186/s13014-017-0830-z.
[7] Pan X, Zhang YH, Li XJ, et al. Clinical report of two dose fractionation regimens using carbon ion radiotherapy for lymph node drainage areas in lung cancer[J]. Chinese Journal of Radiation Oncology, 2023, 32(3): 215-221. DOI: 10.3760/cma.j.cn113030-20220401-00119.
Pan X, Zhang YH, Li XJ, et al. Clinical report of two dose fractionation modes using carbon ion beam therapy in the lymph node drainage area for lung cancer[J]. Chin J Radiat Oncol, 2023, 32(3): 215-221. DOI: 10.3760/cma. j.cn113030-20220401-00119.
[8] Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience[J]. Lancet Oncol, 2015, 16(2):e93-e100. DOI: 10.1016/S1470-2045(14)70412-7.
[9] Miyamoto T, Baba M, Sugane T, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week[J]. J Thorac Oncol, 2007, 2(10): 916-926. DOI: 10.1097/JTO. 0b013e3181560a68.
[10] Saitoh JI, Shirai K, Mizukami T, et al. Hypofractionated carbon-ion radiotherapy for stage I peripheral nonsmall cell lung cancer (GUNMA0701): prospective phase II study[J]. Cancer Med, 2019, 8(15): 6644-6650. DOI:10.1002/cam4.2561.
[11] Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403[J]. Int J Radiat Oncol Biol Phys, 2015, 93(5): 989-996. DOI: 10.1016/j.ijrobp.2015.07.2278.
[12] Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer[J]. JAMA, 2010, 303(11): 1070-1076. DOI: 10.1001/jama.2010.261.
[13] Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study[J]. Lancet Oncol, 2015,16(2):187-199. DOI: 10.1016/S1470-2045(14)71207-0.
[14] Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105[J]. J Clin Oncol, 2010,28(23):3739‐3745. DOI: 10.1200/JCO.2009.24.5050.
[15] Karube M, Yamamoto N, Shioyama Y, et al. Carbon-ion radiotherapy for patients with advanced stage non-small-cell lung cancer at multicenters[J]. J Radiat Res,2017,58(5):761-764. DOI: 10.1093/jrr/rrx037.
[16] Schild SE, Stella PJ, Geyer SM, et al. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in stage III non-small-cell lung cancer[J]. Int J Radiat Oncol Biol Phys, 2002, 54(2): 370-378. DOI:10.1016/s0360-3016(02)02930-9.
