Evaluation of the effect of carbon ion radiotherapy on limited-stage small cell lung cancer
Evaluation of the effect of carbon ion radiotherapy on limited-stage small cell lung cancer
中华肿瘤防治杂志2024年7月第31卷第14期 CHIN J CANCER PREV TREAT, July 2024,Vol. 31 No.14
ZHANG Yihe, ZHANG Yanshan※, YE Yancheng, QIN Tianyan, LI Wanguo, PAN Xin, WANG Xin, YANG Yuling, MA Tong, FENG Lixia
Gansu Wuwei Tumor Hospital, Gansu, Wuwei 733000, China
Abstract: Objective To observe the safety and efficacy of carbon ion radiotherapy combined with concurrent chemotherapy and followed prophylactic cranial irradiation for limited-stage small cell lung cancer. Methods A total of 22 pathologically confirmed limited-stage small cell lung cancer patients admitted to Gansu Wuwei Tumor Hospital from May 27, 2020 to April 13, 2023 were selected as the study objects. The prescribed dose of carbon ions was 72 Gy (RBE)/12 fractions. Carbon ion radiotherapy was used in all patients concurrent with EP regimen chemotherapy. Patients who achieved complete or partial response after carbon ion therapy were given prophylactic cranial irradiation. The level of neuron-specific enolase, treatment-related toxicity, 1-year and 2-year local control rate, progression-free survival rate, and overall survival rate were observed before and after treatment. Results Preliminary result of this pilot study showed 21 of the 22 patients completed concurrent chemoradiotherapy. One patient did not receive chemotherapy due to comorbidity of cardiorenal syndrome; the other patients were well tolerated. The average NSE levels of 22 patients before and after treatment were (30.73±13.27) and (9.56±3.94) ng/ml, respectively, with statistically significant differences (t=7.168, P<0.001). Follow-up period was 5–35 months.
Keywords: Carbon ion therapy; Limited-stage small cell lung cancer; Safety; Concurrent chemoradiotherapy; Prophylactic cranial irradiation
CLC number: R734.2 Document code:A Article ID:1673-5269(2024)14-0887-06
Small cell lung cancer (SCLC) accounts for 15% of all lung cancers and is characterized by rapid growth, high vascular density, genomic instability, early metastasis, and frequent inactivation of TP53 and RB1 genes, leading to high risks of local progression and distant metastasis [1-2]. Although initial treatment achieves high response rates, patients often experience relapse and metastasis, resulting in a low 5-year survival rate [3]. The median survival of limited-stage SCLC (LS-SCLC) patients after systemic therapy is only 1–3 years [4].According to the NCCN 2023 (v1) guidelines for LS-SCLC, Stage I–IIa patients may undergo surgical resection plus adjuvant systemic therapy, stereotactic ablative radiotherapy (SABR) plus adjuvant systemic therapy, or concurrent chemoradiotherapy (CCRT). For Stage IIb–IIIc, CCRT is recommended based on performance status [5]. Meta-analyses indicate that thoracic radiotherapy in LS-SCLC reduces local tumor volume by 25–30% and improves 2-year overall survival (OS) by 5–7% compared to chemotherapy alone [6].The CONVERT trial, a phase 3 randomized study in 547 LS-SCLC patients, compared accelerated hyperfractionation with conventional fractionation, showing similar median OS but higher grade 4 neutropenia in the accelerated group [7]. Proton combined with carbon ion therapy in LS-SCLC demonstrated 2-year OS, local progression-free survival (LPFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) rates of 81.7%, 66.7%, 53.6%, and 41.2%, respectively, with no grade 4–5 toxicities, confirming its safety and efficacy [8-9].However, despite improved OS with radiotherapy, recurrence remains common. Systematic analyses suggest that higher biological effective dose (BED) correlates with longer median OS, better PFS, and improved local control [10]. This study evaluates the safety and efficacy of carbon ion therapy in LS-SCLC, providing clinical insights for its management.
1. Objectives and Methods
1.1 Case Selection and General Information
Twenty-two patients with SCLC who underwent carbon ion therapy at the Heavy Ion Center of Wuwei Tumor Hospital in Gansu Province from May 27, 2020, to April 13, 2023, were selected. They were pathologically diagnosed with LS-SCLC before treatment through enhanced chest CT, positron emission tomography-computed tomography (PET-CT), and enhanced cranial MRI. Their KPS scores were ≥70, and their performance status (PS) scores were ≤2. Among them, there were 4 males and 18 females; the age ranged from 41 to 77 years, with an average age of (62.09±2.15) years and a median age of 62 years. Inclusion criteria: AJCC (American Joint Committee on Cancer) 8th edition staging of stage I-IIIc; age ≥14 years; expected survival time ≥6 months; able to tolerate carbon ion therapy. Exclusion criteria: severe or potentially radiotherapy-affecting comorbidities; prior radiotherapy with a plan for retreatment that cannot meet the dose constraints for critical organs; metal or prosthetic implants that may affect the radiation target dose and cause adverse consequences. The study was approved by the hospital's Medical Ethics Committee (2020-Ethics Review-25) and registered in the Chinese Clinical Trial Registry (ChiCTR2100052807). All patients were informed and signed written informed consent forms.
1.2 Irradiation Method
Patients were placed in the supine or prone position, with their hands crossed over their heads, the left hand on top, and a pillow under the occiput. Double fixation was performed using a large vacuum pad and a thermoplastic film. The CT scan slice thickness was 3 mm. Plain scans, enhanced sequences, and 4DCT scans (Siemens SOMATOM-Definition AS) were performed. The post-scan CT images were transmitted to the "rtstation" planning system for target delineation.
Target Delineation: The Gross Tumor Volume (GTV) included lesions detectable by physical examination, primary lesions, and metastatic lymph nodes visible on CT and PET-CT. The Clinical Target Volume (CTV) included the GTV plus the lymphatic drainage area (ipsilateral hilar, subcarinal, and mediastinal regions). The Internal Target Volume (ITV) was defined as the CTV plus the range of the Maximum Intensity Projection (MIP) from 4DCT. The Planning Target Volume (PTV) 1 was ITV plus 0.5 cm; PTV 2 was GTV plus the MIP range plus 0.5 cm. The prescribed dose was delivered to PTV 1: 48 Gy(RBE)/12 fractions, 4 Gy(RBE)/fraction, and PTV 2: 24 Gy(RBE)/12 fractions, 2 Gy(RBE)/fraction, using Simultaneous Integrated Boost (SIB), with treatment administered 5 days per week. It was required that 95% of the prescribed dose cover 90% of the PTV. The normal tissue dose constraints were referenced from the Chinese Ion Therapy Guidelines (2020 Edition) formulated by the Radiation Oncology Physicians Branch of the Chinese Medical Doctor Association: spinal cord Dmax < 45 Gy(RBE), heart Dmax < 72 Gy(RBE), lung Dmean < 14 Gy(RBE), V20Gy(RBE) < 20% (unilateral), V5Gy(RBE) < 40% (unilateral), esophagus Dmax < 60 Gy(RBE) [11].
Plan Creation: The Wuwei Carbon Ion Planning System (ciPlan1.0) used the Mixed Beam Linear Quadratic Model (MBM-LQ) for RBE calculation. The LQ model parameters obtained from HSG cell survival experiments were utilized to derive the RBE depth distribution curve at the 10% cell survival level, which was used for range modulation device design and plan optimization. To ensure the planned target volume received a uniform biologically effective dose, the planning system used an iterative optimization algorithm to determine the irradiation dose weights for each Bragg peak in carbon ion irradiation. Based on the irradiation weights of each Bragg peak, the physical dose distribution could be obtained. Similar to the Japanese MKM model, in the Wuwei ciPlan RBE model: Clinical Dose = Biological Effective Dose × 1.43, Biological Effective Dose = Physical Absorbed Dose × RBE. Under the same clinical dose, the difference in physical absorbed dose between MKM and ciPlan was minimal. After plan approval, it was transmitted to the ciTreat Treatment Control System (ciTreat V2.0), which used passive or spot scanning technology. The carbon ion beam energy range of 120–400 MeV/u was selected based on the patient's tumor depth and calculation results.
1.3 Other Treatments
Carbon ion therapy was carried out simultaneously with chemotherapy. The chemotherapy regimen was EP: Etoposide (produced by Qilu Pharmaceutical Co., Ltd., batch number AB1K3011) 100 mg/m² on days 1 - 3, and Cisplatin (produced by Qilu Pharmaceutical Co., Ltd., batch number EA4A3008B) 75 mg/m² on day 1, with one cycle every 21 days. It was recommended to continue chemotherapy for 4 cycles after the completion of carbon ion therapy. Efficacy evaluation was conducted after the end of carbon ion therapy. According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, patients with complete response (CR) and partial response (PR) underwent prophylactic cranial irradiation (PCI), with an irradiation dose of 25 Gy(RBE)/10 fractions.
1.4 Observation Indicators
The observation indicators included treatment-related toxicity, local control rates at 1 and 2 years, progression-free survival (PFS), and overall survival (OS). The evaluation of radiation toxicity was based on the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) radiation injury grading system, which assesses acute radiation reactions in different parts of the human body after radiotherapy and is classified into grades 0 - 4. Local control (LC), PFS, and OS at 1 and 2 years were calculated from the start of carbon ion therapy until the occurrence of relevant events or the last follow-up.
The levels of neuron-specific enolase (NSE) in patients were measured before and after treatment. 4 mL of peripheral blood was drawn from patients before and after treatment respectively, and the Neuron-Specific Enolase Assay Kit (produced by Shenzhen New Industries Biomedical Engineering Co., Ltd., batch number 02323041) was used for detection.
1.5 Follow-up
All patients were followed up by phone or WeChat, and relevant data were collected by mail. During the treatment period, patients' blood routine, liver and kidney functions were monitored weekly. In the first year after the end of treatment, patients were re-examined every 3 months, during which physical examinations, blood routine and biochemical tests, tumor markers, abdominal ultrasound, and lung CT were performed. Cranial MRI was arranged if necessary. Re-examinations were conducted every 6 months in the 2nd - 3rd years and every 12 months in the 4th - 5th years. The last follow-up was on September 30, 2023.
1.6 Statistical Methods
SPSS 22.0 was used for statistical analysis of the data. Measurement data were expressed as X ± S, and the t-test was used for comparisons between two groups; counting data were expressed as rates or proportions. The Kaplan-Meier method was used for calculating overall survival rate and progression-free survival rate. The significance level α was set at 0.05 (two-tailed).
2. Results
2.1 Safety
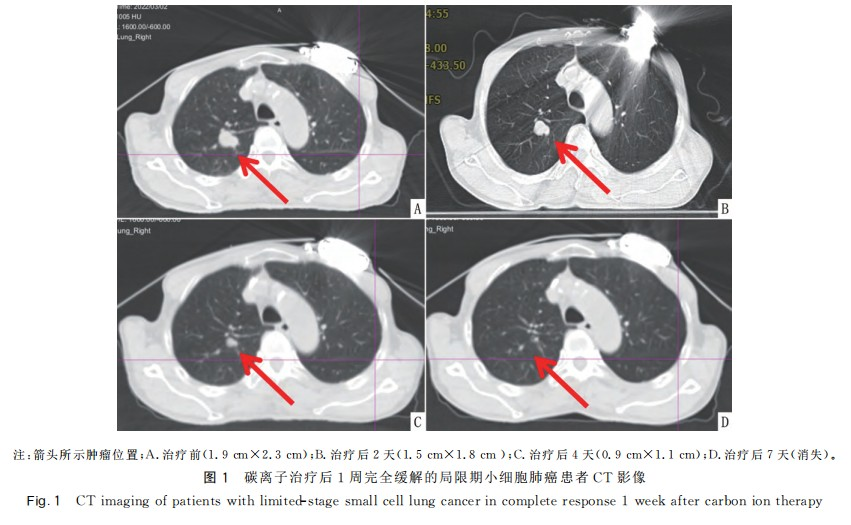
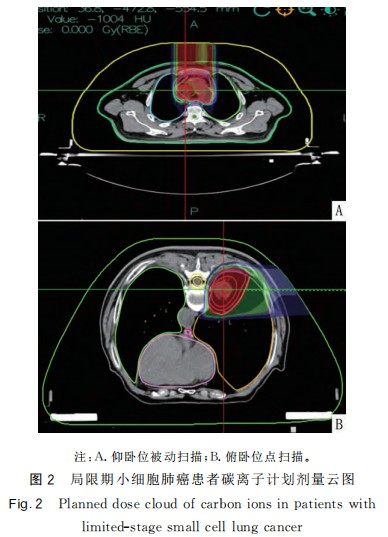
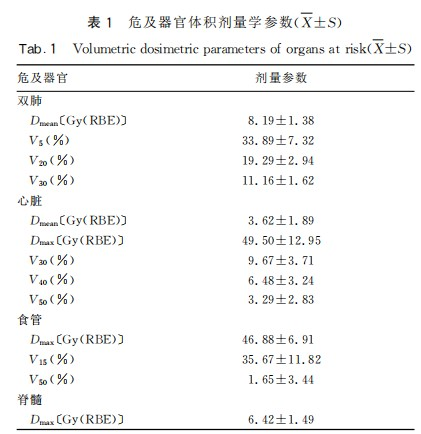
After the completion of carbon ion therapy, there were 7 cases of complete response (CR) and 15 cases of partial response (PR). Imaging of CR patients is shown in Figure 1. Among the 22 patients, only 1 patient was not recommended for chemotherapy due to dilated cardiomyopathy combined with nephrotic syndrome after multidisciplinary consultation. This patient underwent only carbon ion therapy and prophylactic cranial irradiation (PCI). The remaining 21 patients completed carbon ion therapy and concurrent EP chemotherapy as planned. The toxic and side effects during radiochemotherapy were tolerable for the patients. The mean lung dose (Dmean) of both lungs and the heart were both <11 Gy(RBE). The V5, V20, and V30 of both lungs were <48%, 26%, and 14% respectively. The maximum dose (Dmax) of the heart was <71 Gy(RBE), and the V30, V40, and V50 were <16%, 11%, and 9% respectively. The maximum dose (Dmax) of the esophagus was <53 Gy(RBE); the maximum dose (Dmax) of the spinal cord was <9 Gy(RBE). The organ at risk doses in the carbon ion therapy plan are shown in Figure 2 and Table 1.
2.2 Adverse Reactions
Twenty-one patients tolerated radiochemotherapy well and completed at least two cycles of concurrent radiochemotherapy. Among them, there was 1 case (grade 1) of skin reaction, 11 cases (9 cases of grade 1 and 2 cases of grade 2) of pneumonia, 4 cases (grade 1) of esophagitis, 8 cases (5 cases of grade 1, 2 cases of grade 2, and 1 case of grade 3) of leukopenia, 6 cases (4 cases of grade 1, 1 case of grade 2, and 1 case of grade 3) of neutropenia, 2 cases (grade 1) of thrombocytopenia, and 3 cases (grade 1) of anemia. The adverse reactions related to carbon ion therapy included radiation pneumonia (9 cases of grade 1 and 2 cases of grade 2), esophagitis (4 cases of grade 1), and skin reaction (1 case of grade 1), all of which were acute adverse reactions of grades 1 and 2.
2.3 Efficacy
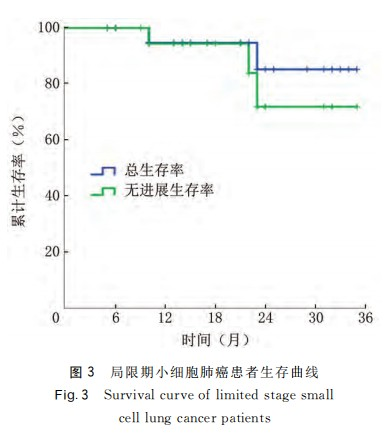
The average levels of neuron-specific enolase (NSE) before and after treatment in 22 patients with LS-SCLC were (30.73 ± 13.27) ng/mL and (9.56 ± 3.94) ng/mL, respectively, and the difference was statistically significant (t = 7.168, P < 0.001). During a follow-up period of 5 - 35 months, with a median follow-up time of 20 months, 1 patient refused prophylactic cranial irradiation (PCI) after carbon ion radiotherapy. No local recurrence or distant metastasis was observed until the last follow-up. Among the remaining 21 patients, no local recurrence or distant metastasis was found during the 1-year follow-up. At the 2-year follow-up, 1 patient experienced local recurrence 16 months after treatment, and 1 patient developed bone metastasis 23 months after treatment. Using Kaplan-Meier analysis, the 1-year and 2-year local control rates of the 22 patients were 100.00% and 92.31%, respectively; the progression-free survival rates at 6, 12, 18, and 24 months were 100.00%, 94.74%, 94.74%, and 77.73%, respectively; the overall survival rates at 6, 12, 18, and 24 months were 100%, 94.12%, 94.12%, and 84.71%, respectively. See Figure 3.
Discussion
Thoracic radiotherapy is an important component in the treatment of LS-SCLC. A study by Morimoto et al. [12] showed that the time from the start of any treatment to the end of chest irradiation (SER) is the most important factor affecting the survival of LS-SCLC. If SER < 30 days, the 5-year survival rate will exceed 20%. A systematic review reported that local recurrence (LR) is the main factor related to the survival of LS-SCLC, and for every 10 Gy (RBE) increase in BED, the local recurrence rate can be reduced by 5.5%, indicating that patients receiving high-BED treatment can reduce the local failure rate and thus improve overall survival (OS) [13]. Therefore, to improve the survival benefits and local control rate of LS-SCLC patients, radiotherapy should be started as early as possible and a higher radiation dose should be delivered in a shorter period.
Carbon ion beams belong to high linear energy transfer (LET). When the beam passes through matter, it forms a low-dose flat region in the shallow part, and when the ions reach the end of their range, their speed drops instantaneously, and the residual energy is completely released, thus forming a high ionization density peak, namely the Bragg peak [14]. By adjusting the energy, the Bragg peak position can be made to reach the high-dose distribution in the tumor area, achieving maximum killing of the tumor without affecting nearby normal tissues [15]. Due to its unique physical properties, at the center rear edge of the carbon ion beam, 5 mm away from the 95% isodose line, the dose can drop to about 20% - 30% of the prescribed dose. Therefore, radiotherapy can be safely performed for patients whose tumors are close to organs at risk [16]. In this study, all 22 included LS-SCLC patients were treated with carbon ion therapy and received concurrent chemotherapy. Utilizing the physical and biological advantages of carbon ions, even when a treatment dose of 72 Gy (RBE)/12 fractions was given to the patients, the doses to the organs at risk were all within the limits. During the treatment period, the patients only experienced grade 1 and 2 carbon ion therapy-related esophagitis, pneumonia, and dermatitis, which were relieved after symptomatic treatment. During the follow-up until 2 years later, 2 patients still had intermittent dry cough, which was considered grade 1 late radiation pneumonia, and no grade 2 or higher late adverse reactions occurred. No carbon ion therapy-related reactions that affected normal life occurred during the carbon ion therapy period and the 2-year follow-up after treatment in all patients. The patients had good tolerance and comfort.
The incidence of radiotherapy-related adverse reactions is related to the radiation dose to organs at risk. Studies have shown that the incidences of grade ≥ 3 pneumonia and esophagitis in lung cancer patients after receiving photon radiotherapy combined with chemotherapy are 4.1% - 10% and 7% - 14%, respectively [17 - 19]. A long-term follow-up study of breast cancer patients after thoracic radiotherapy found that the incidence of coronary events was significantly related to a heart Dmean ≥ 10 Gy (RBE) or higher doses [18]. Shirai et al. [19] proposed in the dose factor analysis of pleural effusion after radiochemotherapy for esophageal cancer that heart V50 is the strongest predictor of pleural effusion occurrence. The incidences of pleural effusion when heart V50 < 20%, 20% ≤ heart V50 < 40%, and heart V50 ≥ 40% are 6%, 44%, and 64%, respectively. Tonison et al. [20] conducted a retrospective analysis of radiation pneumonia after intensity-modulated conformal radiotherapy (IMRT) for esophageal cancer, showing that lung V20 is considered a risk factor for radiation pneumonia occurrence. Lung V20 < 23% can control the incidence of radiation pneumonia within 10%. Watkins et al. [21] found that esophageal V15 is a related factor for grade 3 esophagitis in patients receiving 45 Gy (RBE)/30 fractions of hyperfractionated radiotherapy combined with chemotherapy. When V15 < 60%, the incidence of grade 3 esophagitis is 15%; when V15 ≥ 60%, the incidence of grade 3 esophagitis is as high as 64%. In this study, the patient's heart Dmean < 8 Gy (RBE), D2 < 49 Gy (RBE), V50 < 9%; both lungs Dmean < 10 Gy (RBE), V20 < 26%, V30 < 14%; esophagus Dmean < 53 Gy (RBE), V15 < 43%; spinal cord Dmax < 9 Gy (RBE). The results of this study show that using carbon ion therapy for LS-SCLC, combined with chemotherapy and PCI, has relatively mild adverse reactions. During the treatment process, 1 patient experienced grade 3 acute hematological toxicity, which was considered to be caused by the concurrent chemotherapy drugs. After symptomatic treatment, the patient returned to normal and it did not affect the next cycle of chemotherapy.
In the INT0096 study, the recommended standard photon radiotherapy regimen for SCLC is 45 Gy (RBE)/1.5 Gy (RBE)/2 fractions combined with concurrent EP chemotherapy. The 1-year overall survival rate is 78%, and the local recurrence rate is 36% [22]. You Jing et al. [23] used the IMRT synchronous incremental irradiation method for 26 LS-SCLC patients, and the 1-year overall survival rate and progression-free survival rate were 89% and 51%, respectively. Studies have shown that for stereotactic ablative radiotherapy (SABR) with a dose of 50 Gy (RBE)/5 fractions, the 1-year and 3-year local control rates are 97.4% and 96.1%, respectively, and the 1-year and 3-year overall survival rates are 69.9% and 34%, respectively [24]. In the experiment by Mohamad et al. [25], 30 LS-SCLC patients received proton radiotherapy of 45 - 66 CGE/33 - 37 fractions, with a median overall survival (OS) of 28.2 months, and the 1-year and 2-year overall survival rates were 72% and 58%, respectively.
Carbon ions have unique biological advantages. They can cause about 70% of DNA molecules to have more than two double-strand breaks, which are difficult to repair, resulting in lethal damage to tumor cells. Moreover, this is not dependent on the cell cycle and oxygen environment, and has a relatively high relative biological effect [26 - 28]. In this study, the NSE levels of all 22 patients treated with carbon ions returned to normal levels at the end of treatment; among them, in 1 patient who did not receive concurrent chemotherapy due to renal insufficiency, the tumor completely disappeared within one week after only carbon ion therapy; in 1 patient who refused PCI, brain metastasis occurred 10 months after treatment. At the last follow-up, this patient had no local recurrence or distant metastasis. The results of this study show that the 1-year local control rate, progression-free survival rate, and overall survival rate of LS-SCLC patients are 100.00%, 94.74%, and 94.12%, respectively; the 2-year local control rate, progression-free survival rate, and overall survival rate are 92.31%, 77.73%, and 84.71%, respectively. It can be seen that carbon ion radiotherapy combined with chemotherapy and PCI not only increases the target area dose without increasing the radiation dose to organs at risk, but also has good tolerance and compliance in patients even when combined with concurrent chemotherapy and PCI. Therefore, carbon ion radiotherapy combined with chemotherapy and PCI is safe and effective and may be an optional treatment method for LS-SCLC. This study still has limitations. The number of included cases is relatively small, and the follow-up time is not long enough. More prospective studies and longer-term follow-up observations are needed for research reporting.
Conflict of Interest:None
References
[1] Diao Wenyu, Liu Qing, Meng Linhui, et al. Immunotherapy for a case of extremely elderly small cell lung cancer with chronic renal failure and literature review [J]. Practical Geriatrics, 2023, 37(4): 422 - 423.
[2] Ganti AK,Zhen W,Kessinger A.Limited-stage small-cell lung cancer:therapeutic options[J].Oncology(Williston Park),2007,21(3):303-312.
[3] Parisi S,Ferini G,Lillo S,et al.Stereotactic boost on residual disease after external-beam irradiation in clinical stage III non-small cell lung cancer:mature results of stereotactic body radiation therapy postradiation therapy(SBRT postRT)study[J].Radiol Med,2023,128(7):877-885.
[4] Xu Meiying, Luo Jiawei, Xu Ruilian. Research progress in the treatment of NSCLC with EGFR exon 20 insertion mutations [J]. Chinese Journal of Lung Cancer, 2023, 26(2): 151 - 157.
[5] National Comprehensive Cancer Network.The NCCN clinical practice guidelines in oncology,Small cell lung cancer(version 3.2022)[EB/OL].(2022-05-22)[2024-06-18].https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
[6] Warde P,Payne D.Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung?A meta-analysis[J].J Clin Oncol,1992,19(5-6):146-146.
[7] Faivre-Finn C,Snee M,Ashcroft L,et al.Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer(CONVERT):an open-label,phase 3,randomised,superiority trial[J].Lancet Oncol,2017,18(8):1116-1125.
[8] Grønberg BH,Killingberg KT,Fløtten Ø,et al.High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer:an open-label,randomised,phase 2 trial[J].Lancet Oncol,2021,22(3):321-331.
[9] Ma NY,Chen J,Ming X,et al.Preliminary safety and efficacy of proton plus carbon-ion radiotherapy with concurrent chemotherapy in limited-stage small cell lung cancer[J].Front Oncol,2021,11:766822.
[10] Mori K,Mostafaei H,Sari Motlagh R,et al.Systemic therapies for metastatic hormone-sensitive prostate cancer:network meta-analysis[J].BJU Int,2022,129(4):423-433.
[11] Zhang Q,Kong L,Liu R,et al.Ion therapy guideline(Version 2020)[J].Precis Radiat Oncol,2021,5(2):73-83.
[12] Morimoto M,Okishio K,Akira M,et al.Duration of twice-daily thoracic radiotherapy and time from the start of any treatment to the end of chest irradiation as significant predictors of outcomes in limited-disease small-cell lung cancer[J/OL].Clin Lung Cancer,2017,18(2):e117-e127[2024-05-05].https://www.sciencedirect.com/science/article/pii/S1525730416302297.DOI:10.1016/j.cllc.2016.09.004.
[13] Zhu L,Zhang S,Xu X,et al.Increased biological effective dose of radiation correlates with prolonged survival of patients with limited-stage small cell lung cancer:a systematic review[J/OL].PLoS One,2016,11(5):e0156494[2024-05-05].https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0156494.DOI:10.1371/journal.pone.0156494.
[14] Malouff TD,Mahajan A,Krishnan S,et al.Carbon ion therapy:a modern review of an emerging technology[J].Front Oncol,2020,10:82.
[15] Zhang Yanshan, Ye Yancheng, Zhang Hong. Clinical progress of carbon ion beam radiotherapy for tumors [J]. Science and Technology for Development, 2020, 16(1): 18 - 33.
[16] Ma Xiaoyun, Zhang Yanshan, Zhang Mengling, et al. Analysis of the dose falloff at the edge of the target area in heavy ion radiotherapy [J]. Journal of Oncology, 2021, 27(5): 390 - 394.
[17] Segawa Y,Kiura K,Takigawa N,et al.Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin,vindesine,and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer:OLCSG0007[J].J Clin Oncol,2010,28(20):3299-3306.
[18] Struikmans H,Petoukhova A,Schreur JHM,et al.Is the risk of ischemic heart disease in women after radiotherapy for breast cancer nowadays still(linearly)associated with the mean heart dose?[J].Acta Oncol,2024,10(63):175-178.
[19] Shirai K,Tamaki Y,Kitamoto Y,et al.Dose-volume histogram parameters and clinical factors associated with pleural effusion after chemoradiotherapy in esophageal cancer patients[J].Int J Radiat Oncol Biol Phys,2011,80(4):1002-1007.
[20] Tonison JJ,Fischer SG,Viehrig M,et al.Radiation pneumonitis after intensity-modulated radiotherapy for esophageal cancer:Institutional data and a systematic review[J].Sci Rep,2019,9(1):2255.
[21] Watkins JM,Wahlquist A,Shirai K,et al.Factors associated with severe acute esophagitis from hyperfractionated radiotherapy with concurrent chemotherapy for limited-stage small-cell lung cancer[J].Int J Radiat Oncol Biol Phys,2009,74(4):1108-1113.
[22] Bogart JA,Waqar SN,Mix MD.Radiation and systemic therapy for limited-stage small-cell lung cancer[J].J Clin Oncol,2022,40(6):661-670.
[23] You Jing, Yu Huiming, Song Xiaowei Ma, et al. Phase I/II clinical study of simultaneous integrated boost intensity-modulated radiotherapy for limited-stage small cell lung cancer [J]. Chinese Journal of Lung Cancer, 2017, 20(1): 28 - 34.
[24] Verma V,Simone CB,Allen PK,et al.Multi-institutional experience of stereotactic ablative radiation therapy for stage ismall cell lung cancer[J].Int J Radiat Oncol Biol Phys,2017,97(2):362-371.
[25] Mohamad O,Sishc BJ,Saha J,et al.Carbon ion radiotherapy:a review of clinical experiences and preclinical research,with an emphasis on DNA damage/repair[J].Cancers(Basel),2017,9(6):66.
[26] Zhang Jinhua. The Role of the Wnt/β-catenin Pathway in Regulating the Radiosensitivity of Quiescent He-La Cells to Carbon Ion Beam Irradiation [D]. Beijing: University of Chinese Academy of Sciences, 2022.
[27] Friis I,Verkhovtsev AV,Solov'yov IA,et al.Lethal DNA damage caused by ion-induced shock waves in cells[J].Phys Rev E,2021,104(5-1):054408.
[28] Wang Jiangtao. Research on the Regulatory Effect and Mechanism of Heavy Ion (12C6+) Irradiation on the Immune Microenvironment of Non-small Cell Lung Cancer [D]. Gansu: Lanzhou University, 2022.
