Breaking the Boundaries: Breakthrough Application of Carbon Ion Radiotherapy ...
Breaking the Boundaries: Breakthrough Application of Carbon Ion Radiotherapy in Advanced Non-Small Cell Lung Cancer
Primary Bronchogenic Carcinoma: Current Challenges and Innovative Treatment Strategies
The 5-year survival rate for primary bronchogenic carcinoma remains as low as 19%, primarily due to the mild or overlooked early symptoms in many patients, leading to initial diagnoses at advanced stages.
Lung cancer is broadly classified into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Approximately 30%–40% of NSCLC patients are diagnosed at stage IV (metastatic disease) at initial presentation. Additionally, some non-stage IV patients may rapidly progress to stage IV due to individual variations, with a median survival time of only 3 months for this group.
For stage IV NSCLC patients, current treatment strategies are primarily based on histopathological subtype and driver gene mutation status, integrating chemotherapy, targeted therapy, and immunotherapy. Recent studies suggest that radiotherapy to the primary tumor in advanced NSCLC may prolong survival, particularly in oligometastatic disease, where local ablative therapy can provide additional survival benefits.
In an innovative approach, our hospital’s Radiation Oncology Department II incorporated heavy ion therapy (carbon ion radiotherapy) into the multidisciplinary treatment of a lung cancer patient with brain metastases. The team delivered precision irradiation to both the primary lung lesion and brain metastases using heavy ions, combined with chemotherapy and subsequent immunotherapy, achieving favorable therapeutic outcomes.
This article will systematically review the therapeutic rationale and clinical management of this case, exploring the potential clinical value of heavy ion radiotherapy in the multidisciplinary treatment of advanced non-small cell lung cancer (NSCLC), with the aim of providing evidence-based insights for clinical decision-making in similar patients.
Case Presentation
A 66-year-old male patient with a 7-year history of hypertension, well-controlled on oral nifedipine sustained-release tablets, initially presented on June 11, 2019, with cough and sputum production following a "cold." A chest CT at Sir Run Run Shaw Hospital, Zhejiang University, revealed a right upper lobe pulmonary nodule (33 mm × 36 mm) with invasion of the anterior segmental bronchus and compression of the superior vena cava. Bronchoscopy and biopsy confirmed adenocarcinoma (right lung) . Driver gene testing was negative, and PD-L1 expression was TPS 20%.
From July 15, 2019, the patient received 4 cycles of chemotherapy (pemetrexed + nedaplatin) at Xinjiang Occupational Disease Hospital, with stable disease (SD) as the best response. In July 2020, PET/CT showed progression: a hypermetabolic right upper lobe mass (44 mm × 37 mm, SUVmax 20.4), pleural nodular thickening with increased uptake (SUVmax 6.2, suggestive of pleural metastasis). The treatment was escalated to 3 cycles of pemetrexed + nedaplatin + camrelizumab, achieving partial response (PR). Due to grade III gastrointestinal toxicity, chemotherapy was discontinued, and the patient continued maintenance immunotherapy (camrelizumab monotherapy for 4 cycles).
On March 19, 2021, a follow-up chest CT at the Third People’s Hospital of Xinjiang Uygur Autonomous Region demonstrated a lobulated mediastinal soft tissue mass in the right upper lobe, with further invasion into the mediastinum and slight enlargement compared to prior imaging. In April 2021, PET/CT at our hospital revealed:
- A hypermetabolic soft tissue nodule adjacent to the right upper lobe bronchus (consistent with primary lung cancer).
- Multiple small hypermetabolic nodules along the right pleura (suggestive of metastatic disease).
- A crescent-shaped hyperdense nodule in the right parietal lobe with increased metabolism (likely metastatic with hemorrhage).
Brain MRI with contrast confirmed a right occipital lobe lesion with abnormal enhancement, consistent with metastatic involvement and hemorrhage.
Imaging Findings
- June 2019 (Chest CT, Sir Run Run Shaw Hospital, Zhejiang University):Revealed a right upper lobe pulmonary nodule (33 mm × 36 mm) with invasion of the anterior segmental bronchus and compression of the superior vena cava.
- July 2020 (PET-CT, Affiliated Tumor Hospital of Xinjiang Medical University):Demonstrated a hypermetabolic right upper lobe soft tissue mass (44 mm × 37 mm, SUVmax 20.4), highly suggestive of primary lung malignancy. Additionally, nodular pleural thickening with increased metabolic activity (SUVmax 6.2) was observed, consistent with pleural metastasis.
- April 2021 (PET-CT at admission):Showed narrowing and truncation of the right upper lobe anterior segmental bronchus, along with a hypermetabolic soft tissue nodule adjacent to the bronchus, highly suspicious for lung cancer. Multiple small hypermetabolic nodules on the right pleura suggested metastatic involvement. A crescent-shaped hyperdense nodule in the right parietal lobe with elevated metabolism raised concern for brain metastasis.
- December 15, 2022 (Brain MRI):Identified a nodular lesion near the posterior horn of the right lateral ventricle, surrounded by extensive perilesional edema, indicative of metastatic disease.
Bronchoscopy and Pathological Examination
June 2019:
Bronchoscopy with biopsy confirmed the diagnosis of adenocarcinoma (right lung).
Molecular Testing
- Genetic testing: EGFR/ALK/ROS1/RAS/BRAF/MET/NTRK (negative).
- PD-L1 expression: TPS 20%.
Diagnosis
- Malignant neoplasm of right upper lung lobe (Adenocarcinoma, with secondary malignant tumors of brain and pleura; cT4N0M1c, stage IVB; KPS score: 80)
- Hypertension, grade 2 (high-risk)
The diagnosis was confirmed by imaging studies and pathological examination, allowing definitive differentiation from other potential diseases.
Treatment
Following multidisciplinary team (MDT) discussion, the treatment plan was determined as: carbon ion radiotherapy (CIRT) for the right lung cancer, right pleural metastases, and brain metastases, followed by sequential immune checkpoint inhibitor therapy.
Positioning for Thoracic Carbon Ion Radiotherapy:
The patient was placed in the supine position, immobilized using a vacuum cushion with a thermoplastic body mask. Care was taken to ensure that the right-sided carbon ion beam entry path avoided the vacuum cushion. Multiple skin marker lines were drawn to guarantee high reproducibility of positioning between simulation and treatment sessions.
Target Delineation (Figure 1):
Based on serial chest CT and PET-CT imaging:
- GTVa: Defined as the right lung tumor on simulation CT fused with the most recent PET/CT.
- CTVa: Created by expanding GTVa by 0.8 cm.
- PTVa: Expanded CTVa by 5 mm.
- PTVa boost: Expanded GTVa by 5 mm.
- GTVb: Defined as the right pleural lesion on simulation CT fused with the most recent PET/CT, including all previously identified pleural metastases.
- CTVb: Expanded GTVb by 0.5 cm.
- PTVb: Expanded CTVb by 5 mm.
- PTVb boost: Expanded GTVb by 5 mm.
Radiotherapy Parameters:
- 2D uniform scanning carbon ion therapy was employed.
- Beam arrangement: Combined vertical and horizontal fields for target coverage.
- Dose prescription:
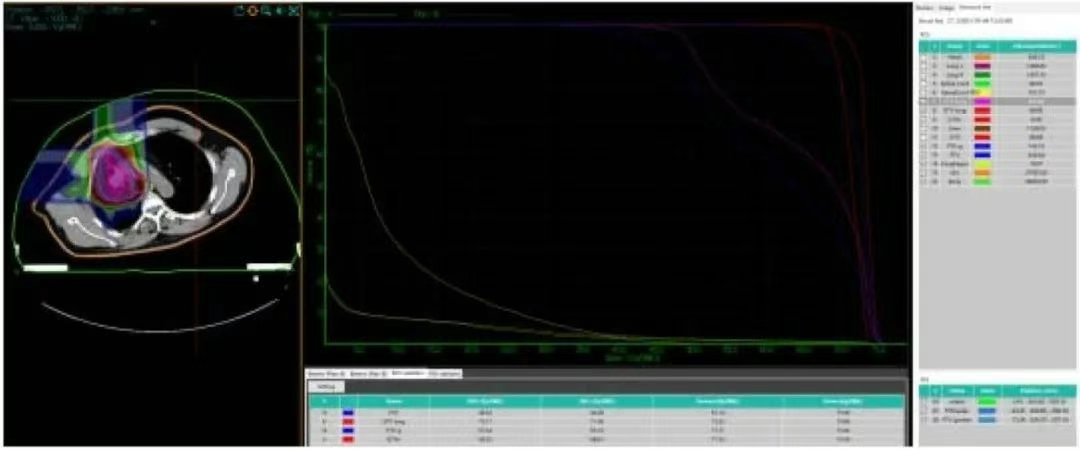
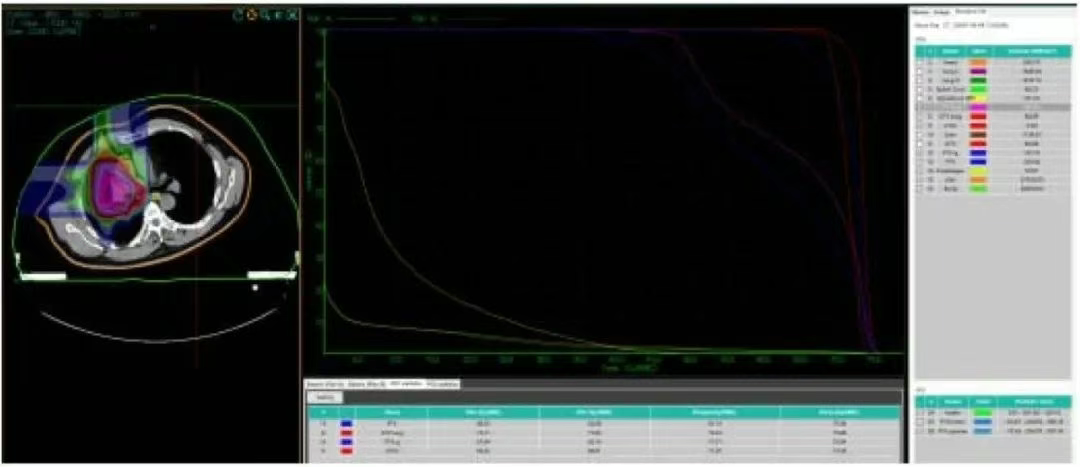
Figure 1. Dose-volume histogram (DVH) of carbon ion treatment planning for the right lung lesion and right pleural metastatic lesions in the patient.
Carbon Ion Radiotherapy for Brain Metastases
Patient Positioning:
The patient was positioned in the supine position, immobilized using a head-neck-shoulder thermoplastic mask with headrest foam. A multi-leaf collimator-based coplanar beam arrangement was planned. Multiple skin markers were applied to ensure precise reproducibility of treatment positioning.
Target Delineation:
- GTVc: Defined as the intracranial oligometastatic lesion identified on simulation CT fused with diagnostic brain MRI
- PTVc: Created by expanding GTVc with a 2 mm margin
Treatment Parameters:
- Technique: Carbon ion therapy using 2D scanning layer (2DSL) uniform scanning
- Beam arrangement: Three coplanar fields
- Dose prescription:
The CiPlan carbon ion therapy planning system is planned to be used to develop the treatment plan. The dose volume histogram (DVH) shows:
1. Chest Plan (Figure 1)
• The 71.06 Gy (RBE) isodose curve encloses 95% of the GTVa. The 71.11 Gy (RBE) isodose curve encloses 90% of the GTV. The 43.3 Gy (RBE) isodose curve encloses 95% of the PTVa. The 46.5 Gy (RBE) isodose curve encloses 90% of the PTVa. The 65.1 Gy (RBE) isodose curve encloses 95% of the PTVa boost. The 67.6 Gy (RBE) isodose curve encloses 90% of the PTVa boost. The 68.6 Gy (RBE) isodose curve encloses 95% of the GTVb. The 69.3 Gy (RBE) isodose curve encloses 90% of the GTVb. The 43.3 Gy (RBE) isodose curve encloses 95% of the PTVb. The 46.5 Gy (RBE) isodose curve encloses 90% of the PTVb. The 65.1 Gy (RBE) isodose curve encloses 95% of the PTVb boost. The 67.6 Gy (RBE) isodose curve encloses 90% of the PTVb boost.
• Left lung dose: Dmean = 1.18 Gy (RBE), V5 = 1.29%, V20 = 0.74%, V30 = 0.08%.
• Right lung - PTV: Dmean = 9.27 Gy (RBE), V5 = 58.42%, V20 = 12.26%, V30 = 7.77%.
• Spinal cord PRV = 16.51 Gy (RBE).
• Heart Dmean = 7.52 Gy (RBE), V30 = 8.36%, V40 = 0%.
2. Head Plan (Figure 2)
• The 69.7 Gy (RBE) isodose curve encloses 95% of the GTV. The 70.52 Gy (RBE) isodose curve encloses 90% of the GTV. The 63.24 Gy (RBE) isodose curve encloses 95% of the PTV. The 65.86 Gy (RBE) isodose curve encloses 90% of the PTV.
• Brain - PTV dose: V50 = 14.92 cc, V60 = 7.67 cc, Dmax = 72.84 Gy (RBE). Brainstem PRV = 0.2 Gy (RBE).
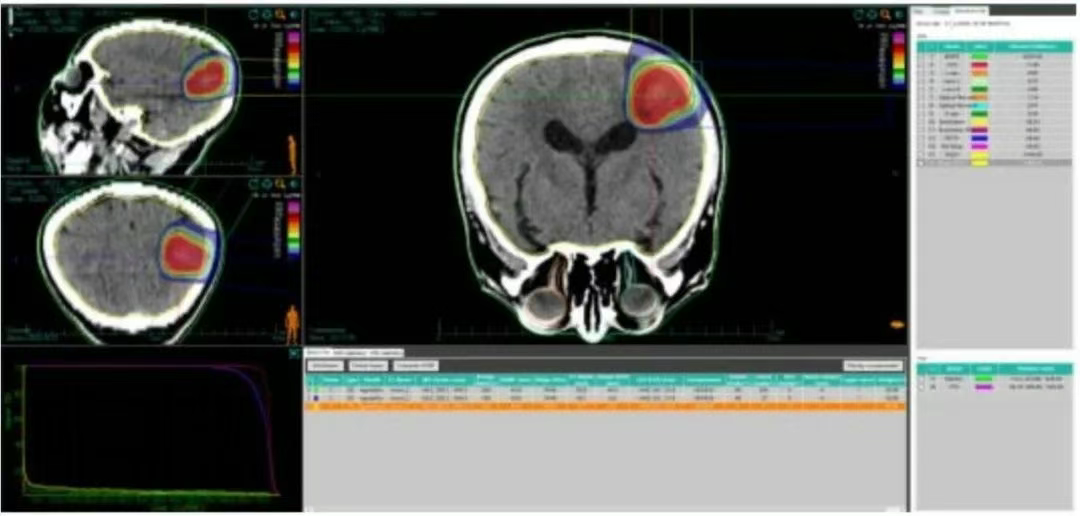
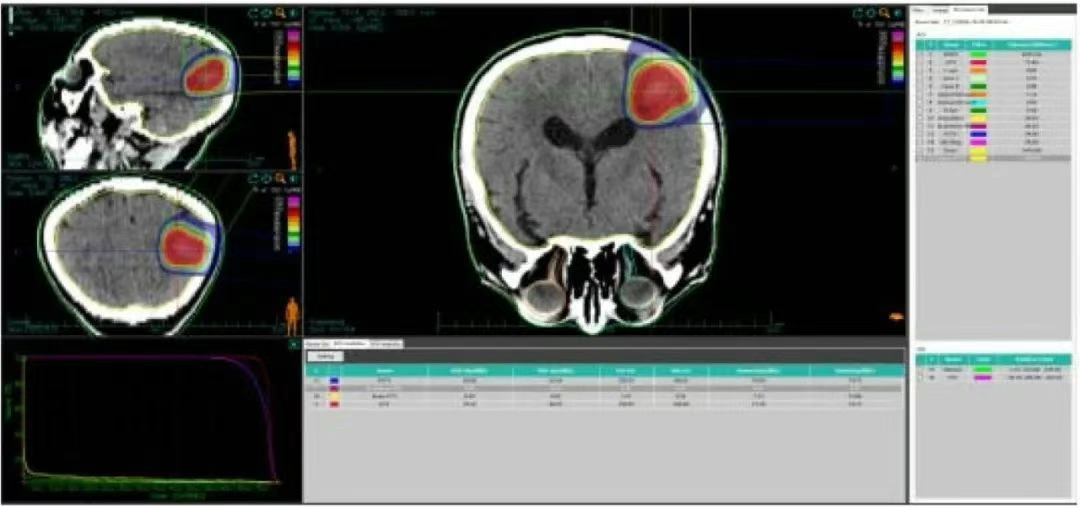
Figure 2 Dose Volume Histogram of Carbon Ion Plan for Patient's Brain Metastatic Lesion
After the treatment plan was approved, dose verification was carried out for carbon ion therapy. The head plan and the chest plan started treatment on the same day. Patient position registration was performed before each carbon ion radiotherapy session. During the treatment process, a low-dose chest CT scan was performed once a week. The treatment plan was adjusted according to the tumor regression. Regular follow-up examinations were conducted after treatment. The treatment efficacy and adverse events were evaluated according to the RECIST 1.1 solid tumor response evaluation criteria and CTCAE 5.0. Radiation injury was assessed according to the RTOG acute radiation injury grading criteria.
After discharge, the patient underwent immunomaintenance therapy with "Tislelizumab 200 mg 1 f/3w" for 10 cycles until January 2023 (the patient stopped the immunomaintenance therapy voluntarily). On December 15, 2022, a head MRI re-examination at the Third Hospital of Xinjiang Uygur Autonomous Region showed: a nodular lesion adjacent to the posterior horn of the right lateral ventricle, surrounded by a large area of edema signal, considered as metastasis.
The patient visited our hospital again on February 2023, complaining of occasional cough and expectoration, without dizziness, headache, nausea, vomiting or other discomforts. A head MRI showed: an abnormally enhanced lesion in the right occipitoparietal lobe. Combined with the MRS findings, it was mostly considered as radiation encephalitis and surrounding edema and glial hyperplasia after metastasis treatment. It was recommended to perform a PET/CT examination for further clarification.
PET/CT showed: Compared with the previous PET/CT before admission (April 20, 2021):
① A patchy soft tissue density shadow adjacent to the mediastinum in the anterior segment of the upper lobe of the right lung, with slightly increased metabolism. The range was significantly reduced compared with before, and the metabolism was decreased. It was mostly considered as activity suppression of lung cancer after treatment or combined with inflammation. Close follow-up was recommended.
② The several small hypermetabolic nodules in the right pleura shown in the previous film were not clearly visible.
③ A large finger-like edema in the right temporal-occipital-parietal lobe, with metabolism lower than that of the contralateral side. The small calcification foci inside and the irregular lace-like slightly hypermetabolic shadow were mostly considered as post-radiation brain changes.
Subsequently, the patient underwent irregular re-examinations. On July 2024, a chest CT at the Third Hospital of Xinjiang Uygur Autonomous Region showed: a lobulated mass adjacent to the right upper mediastinum and atelectasis of the upper lobe, and scattered streaks and patchy shadows in the right lung, with little change compared with before. An abnormal signal adjacent to the posterior horn of the right lateral ventricle on the head MRI, with the range of the lesion and edema reduced compared with before.
Treatment Results and Follow-up
Two months after carbon ion therapy, the patient experienced right-sided chest pain at the entrance site of the carbon ion therapy radiation field, with an NRS score of 5. According to the acute radiation injury grading criteria, it was determined to be grade 2 radiation bone injury, and evaluated as grade 2 chest pain according to CTCAE 5.0. The chest pain subsided 2 months after symptomatic treatment. No other treatment-related adverse reactions were observed. The treatment efficacy was evaluated based on the results of enhanced chest CT and plain and enhanced head MRI examinations. The specific results are shown in Table 1.
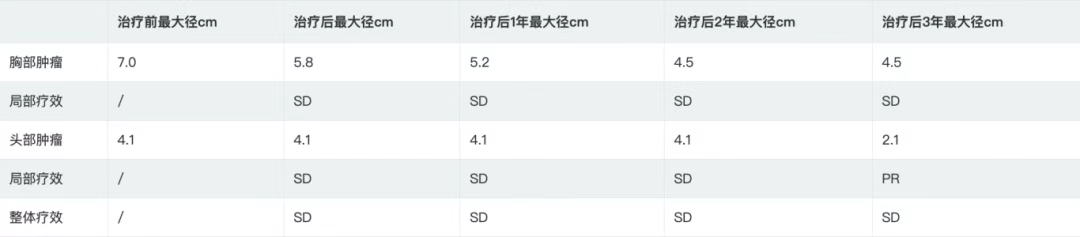
Table 1 Maximum Diameter of Tumors in the Head and Chest before Treatment, after Treatment, and 1, 2, and 3 Years after Treatment
According to the RECIST 1.1 criteria, the treatment efficacy of the chest tumor was SD (Figures 3, 4, 5, and 6). For the head metastases, the efficacy was SD in the first and second years and PR in the third year, with an overall efficacy of SD (Figure 8). However, the PET/CT scan in the second year indicated low metabolism in both the chest and brain lesions (Table 2, Figures 7 and 9). No treatment-related late radiation adverse reactions were observed. Subsequent long-term follow-up for 5 years will continue.

Table 2 Tumor Size and SUVmax of Chest Lesions before and after Treatment
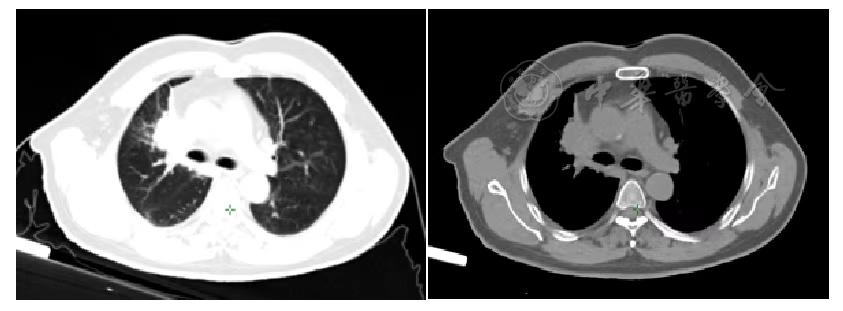
Figure 3 Chest Tumor before Carbon Ion Therapy
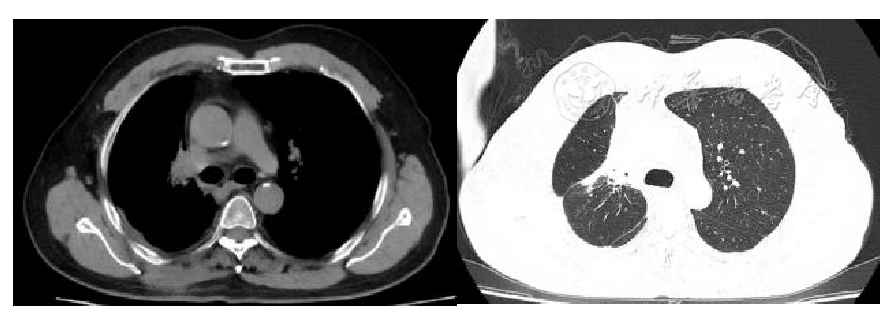
Figure 4 Chest Tumor 1 Year after Carbon Ion Therapy
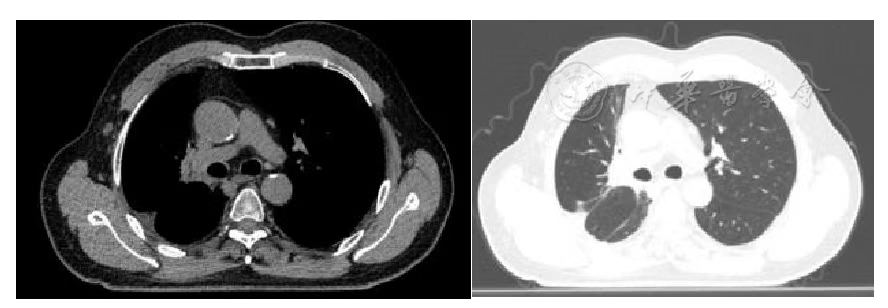
Figure 5 Chest Tumor 2 Years after Carbon Ion Therapy
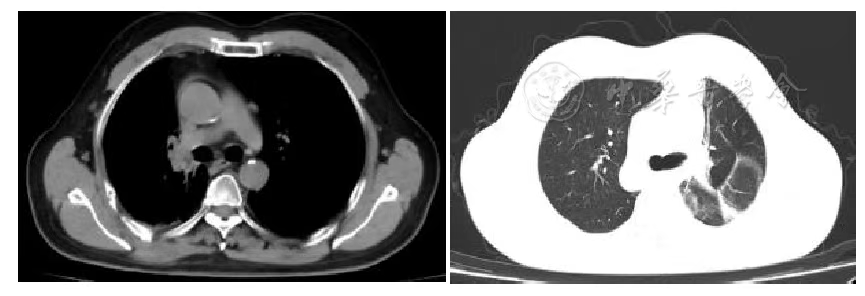
Figure 6 Chest Tumor 3 Years after Carbon Ion Therapy
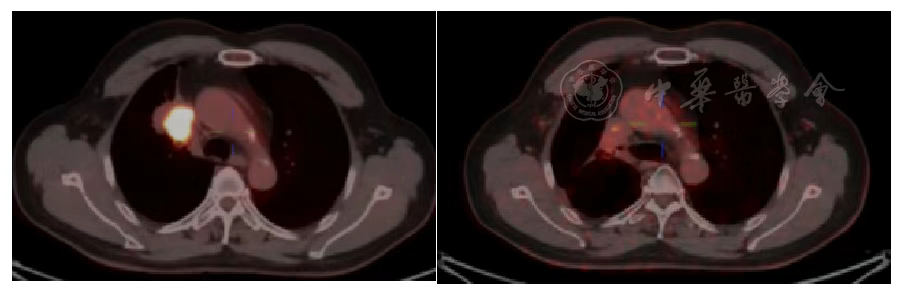
Figure 7 Comparison of Primary Lung Lesion on PET/CT Before and 2 Years After Heavy Ion Therapy
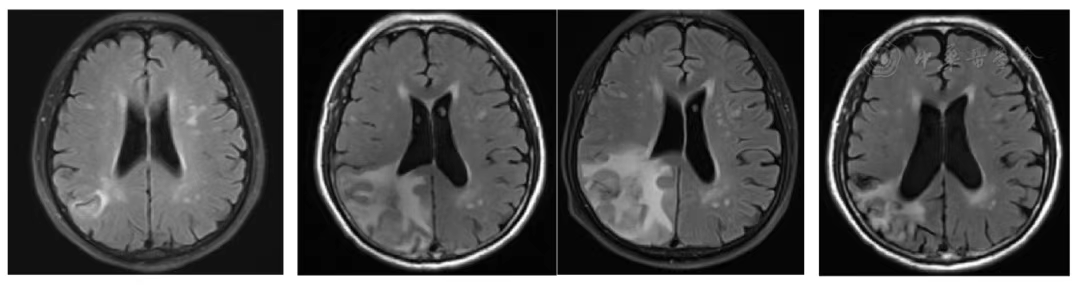
Figure 8 MRI Comparison of Brain Metastases Before and at 1, 2, 3 Years After Heavy Ion Therapy
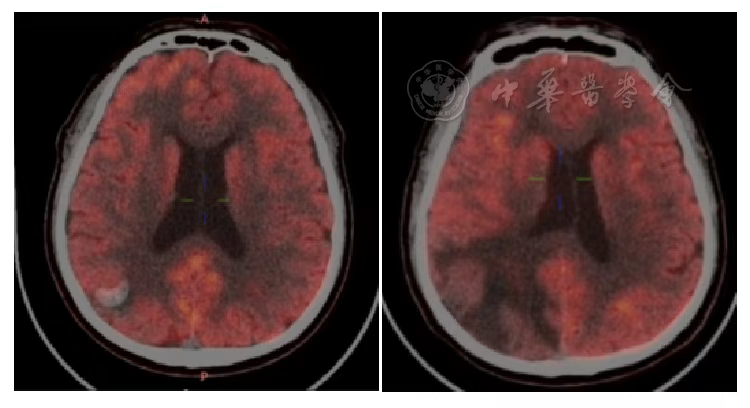
Figure 9 PET/CT Comparison of Brain Metastases Before and 2 Years After Heavy Ion Therapy
Discussion
Lung cancer is one of the most common malignant tumors of the respiratory system, with a high degree of malignancy and a 5-year survival rate of only 19%. According to statistics, in 2020, there were 2.2 million new cases of lung cancer worldwide, accounting for 11.4% of the global cancer incidence, and 1.79 million deaths, representing 18.0% of global cancer mortality—ranking first in mortality and second in incidence after breast cancer.
In 2024, China's National Cancer Center reported 1,060,600 new lung cancer cases and 733,300 deaths in 2022, making it the leading cause of cancer incidence and mortality. Lung cancer is generally classified into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with NSCLC accounting for over 80% of cases (approximately 85% in China). Among NSCLC patients, 30%–40% are diagnosed at stage IV.
For stage IV NSCLC, current treatment strategies—based on pathological type and driver gene mutations—include chemotherapy, targeted therapy, and immunotherapy. Historically, radiotherapy (RT) played only a palliative role, but recent studies suggest that integrating RT into stage IV NSCLC management can improve outcomes. Fairchild et al. analyzed PubMed articles (1966–2007) on RT for advanced NSCLC and found that higher-dose thoracic RT (≥35 Gy) improved survival, with symptom relief enabling systemic therapy and reducing risks like pneumonia or thromboembolism. Fleckenstein et al. reported that radical treatment (RT, surgery, or combined) improved long-term survival in oligometastatic NSCLC patients with good Karnofsky Performance Status (KPS), regardless of synchronous or metachronous metastases.
In 2016, Gomez et al. published a multicenter randomized phase II trial involving 74 NSCLC patients with ≤3 metastatic lesions after first-line systemic therapy. The local consolidative therapy (LCT) group received RT/surgery for all lesions followed by maintenance therapy, while the control group received maintenance therapy alone. At a median follow-up of 12.39 months, LCT significantly prolonged median progression-free survival (mPFS: 11.9 vs. 3.9 months; HR 0.35, P=0.005) with comparable toxicity. Long-term follow-up showed superior overall survival (mOS: 41.2 vs. 17.0 months, P=0.017), supporting LCT for oligometastatic NSCLC.
A retrospective study by Prof. Xu Qinghua et al. analyzed 145 EGFR-mutant (exon 19 del/L858R) NSCLC patients with ≤5 metastases. Patients receiving ablation (RT/surgery) for all lesions had longer mPFS (20.6 vs. 15.6/13.9 months, P<0.001) and mOS (40.9 vs. 34.1/30.8 months, P<0.001) versus partial/no ablation. Grade 3 adverse events included rash (6.2%), elevated ALT (2.7%), radiation pneumonitis (7.7%), and esophagitis (16.9%), with no EGFR-TKI-related interstitial lung disease.
Immunotherapy has recently transformed NSCLC management. A phase I/II trial by Welsh et al. evaluated pembrolizumab ± stereotactic RT in 100 advanced NSCLC patients. Although combined therapy did not significantly improve PFS overall (9.1 vs. 5.1 months, P=0.52), subgroup analysis showed benefit in PD-L1-low patients (20.8 vs. 4.6 months, P=0.004).
Heavy Ion Therapy: Physical and Biological Advantages
Carbon ion radiotherapy (CIRT) offers unique physical and biological benefits. Its Bragg Peak effect delivers maximal energy at the tumor depth while sparing adjacent tissues. Biologically, CIRT induces DNA double-strand breaks, triggering apoptosis and releasing tumor antigens to stimulate immunity. Ran et al. demonstrated that CIRT elevates pro-inflammatory HMGB1 more effectively than X-rays while suppressing IL-10/TGF-β, suggesting superior immunogenicity. The PACIFIC trial (Faivre-Finn et al.) further supports combining immunotherapy with RT, with durvalumab showing 5-year OS of 42.9% vs. 33.4% for placebo.
Clinical Case
Our institution treated a stage IV oligometastatic NSCLC patient (brain/pleural metastases) in March 2020 with CIRT plus concurrent chemotherapy, followed by 9 months of immune checkpoint inhibitor therapy. PET/CT at 2 years revealed no metabolic activity in primary lung, pleural, or brain lesions. Grade 2 chest pain (attributed to pleural CIRT) resolved within 2 months.
Conclusion
CIRT’s precision and immunomodulatory potential make it a promising option for oligometastatic NSCLC, particularly combined with systemic therapies. However, optimal dosing, fractionation, and timing with other modalities require further investigation.
