A Case of Small Cell Lung Cancer: Treatment Course
A Case of Small Cell Lung Cancer: Treatment Course
How many decades does life afford us?
At Wuwei Academy of Medical Sciences Cancer Hospital, there is a patient with advanced lung cancer who has undergone intermittent treatment for a full decade. This journey has deepened our profound understanding of cancer care—and it offers a powerful message to our fellow patients living with cancer:
May you find more courage and hope on this long path of "living with the disease." Stick to standardized treatment, and strive to live lives filled with greater quality, dignity, and hope.
Case Presentation: A 10-Year Survival Journey in Advanced Lung Cancer
In October 2013, our hospital admitted Mr. Yang XX, a local patient diagnosed with lung squamous cell carcinoma. He presented to our Thoracic Surgery Department with "irritative dry cough and shortness of breath." Chest CT revealed a 42mm×35mm×32mm space-occupying lesion in the anterior mediastinal thymus region, accompanied by multiple nodular lesions in the right lung and destructive bone changes in the 12th thoracic vertebra. Head and lumbar spine MRI showed no obvious tumorous lesions.
In early November 2013, he underwent a right thoracotomy exploration under general anesthesia. Pathological examination confirmed: (Mediastinal) poorly differentiated squamous cell carcinoma of the lung, with cancerous infiltration in the 3rd rib and diaphragmatic tissue.
Post-surgery, he received 3 cycles of chemotherapy, and follow-up scans showed lesion shrinkage. However, due to financial constraints, he discontinued further specific treatment. On November 1, 2016, a repeat chest CT indicated disease progression (PD). Synchronous radiochemotherapy was recommended, but he and his family opted for 6 cycles of intermittent chemotherapy instead.
From June to October 2018, he received 4 more chemotherapy cycles due to renewed progression. Follow-up CT showed stable disease (SD), but he again halted treatment for financial reasons.
From November 9, 2018, to September 2019, Mr. Yang participated in a department-led clinical trial, receiving Compound Hongdoushan Capsules 0.6g orally three times daily. Regular monitoring confirmed continued SD. In August 2019, however, CT revealed PD, and he withdrew from the trial.
Between July 2020 and January 2023, he received intermittent chemotherapy at our hospital. Poor treatment compliance led to irregular cycles, and in December 2021, he developed brain metastases. He underwent two-dimensional conformal radiotherapy for brain metastases from December 16 to 28, 2021.
In January 2023, his condition progressed again due to non-adherence to chemotherapy. He switched to oral afatinib maleate tablets (40mg once daily, self-provided) for targeted therapy—continuing to this day. On March 3, 2023, follow-up plain CT scans of the chest/abdomen/pelvis and brain MRI showed SD on efficacy evaluation.
Current Status & Reflection
Ten years later, Mr. Yang remains in good general condition: he lives and works normally, his family is healthy, and he enjoys time with his grandchildren. Every encounter with him reminds us: Life is long—what matters most is happiness and living with purpose.
Our department will continue to follow up with Mr. Yang. We wish every patient a high-quality, hopeful life—where treatment and resilience bring peace and joy.
Treatment-Related Imaging Data
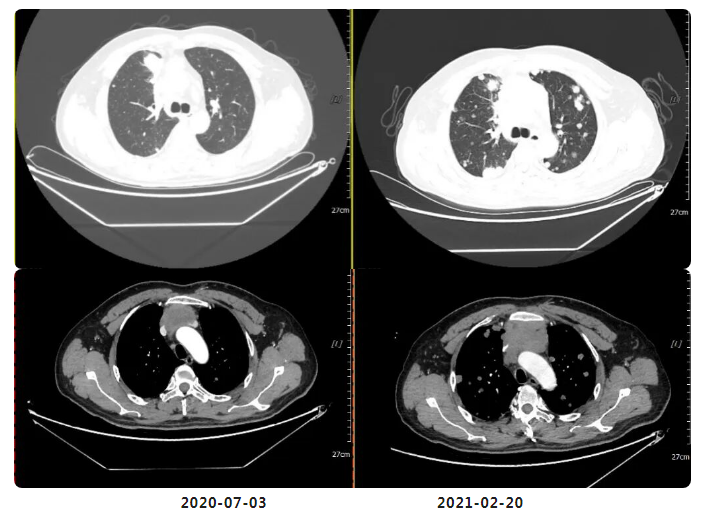
Compared with the imaging studies on July 3, 2020:
Multiple abnormal nodules in both lungs have significantly increased in number and size (previously ~3.2×2.1 cm, now ~3.9×3.0 cm).
Multiple enlarged lymph nodes in the anterior mediastinal space, mediastinum, and bilateral hilar regions have partially grown larger (previously ~5.2×3.4 cm, now ~6.7×4.0 cm), with newly noted sternal involvement. Additionally, adjacent superior vena cava (SVC) and left brachiocephalic vein (LBCV) show worsened involvement compared to prior scans.
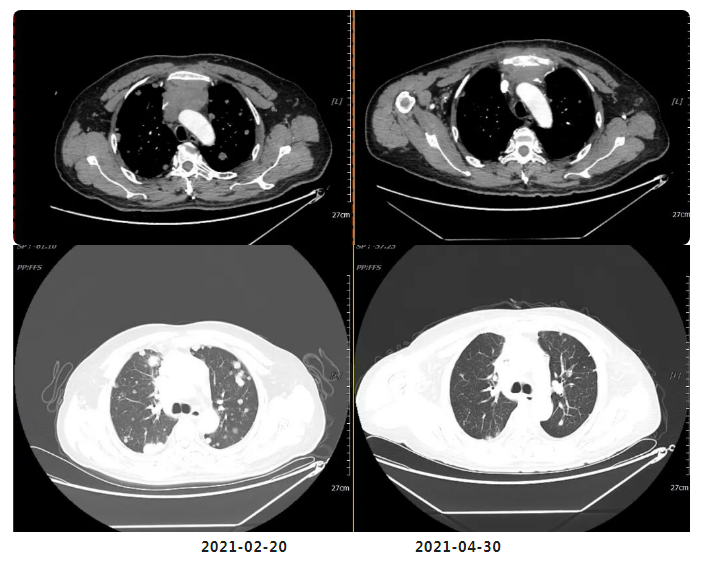
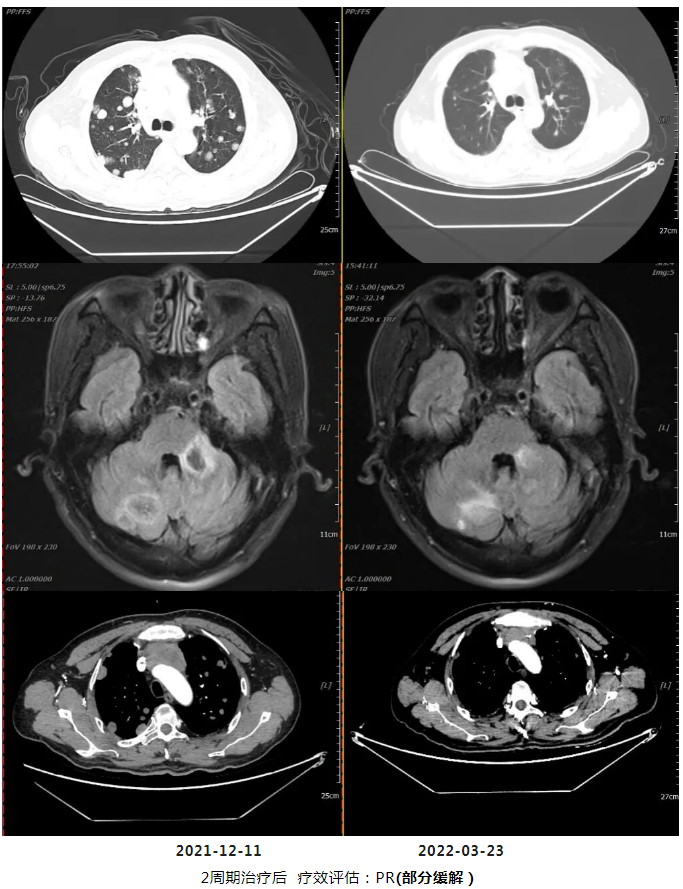
Efficacy Evaluation After 2 Cycles of Chemotherapy: PR
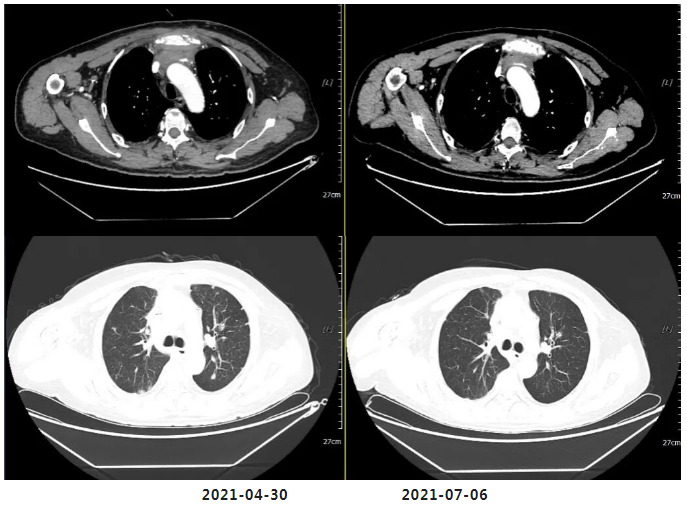
(After 4 Cycles of Chemotherapy) Efficacy Evaluation: SD
(Reduction and shrinkage of nodules in both lungs and pleura; partial reduction in enlarged lymph nodes in the anterior mediastinal space, mediastinum, and bilateral hilar regions—now measuring 2.5×3.0 cm, down from prior assessments.)
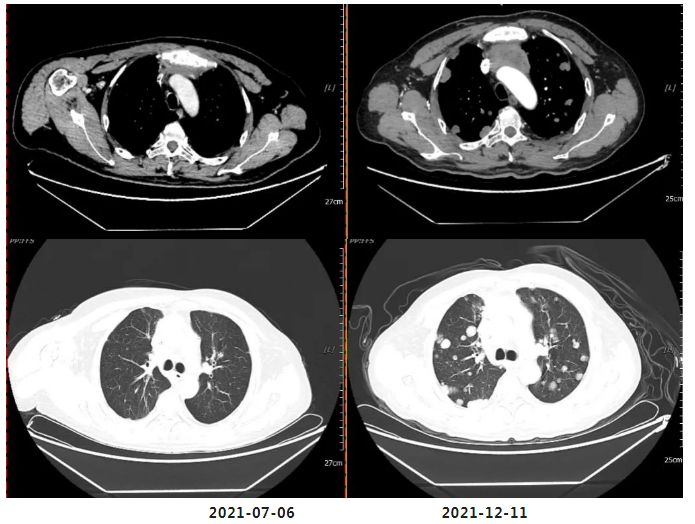
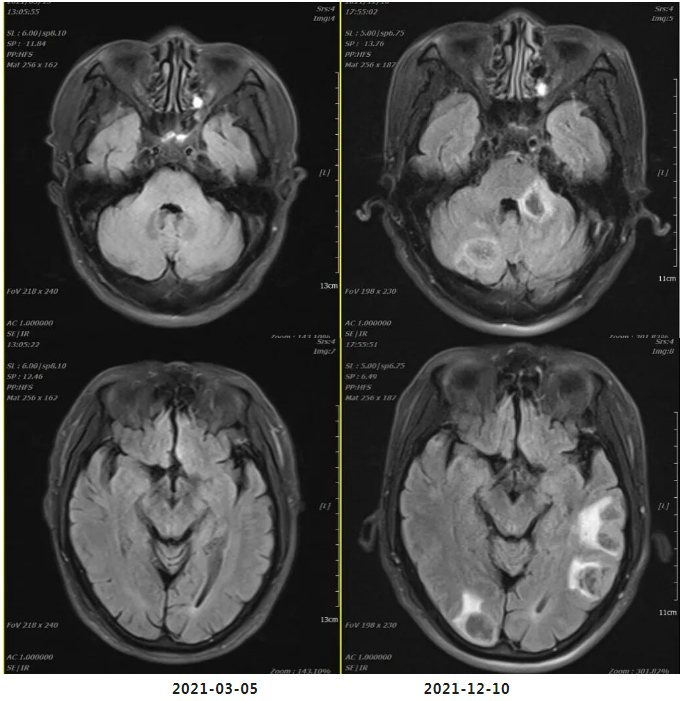
Efficacy evaluation on December 11, 2021: PD (Progressive Disease). Findings include: significant increase in the number and size of nodules in both lungs and pleura; metastatic lymph nodes in the anterior mediastinal space, within the mediastinum, and at bilateral lung hilums have increased in number and size compared to prior assessments, now measuring approximately 4.7×3.5 cm; involvement and stenosis of the superior vena cava and left brachiocephalic vein; newly developed metastases in both cerebellar hemispheres and cerebral hemispheres (approximately 2.1×1.7 cm).
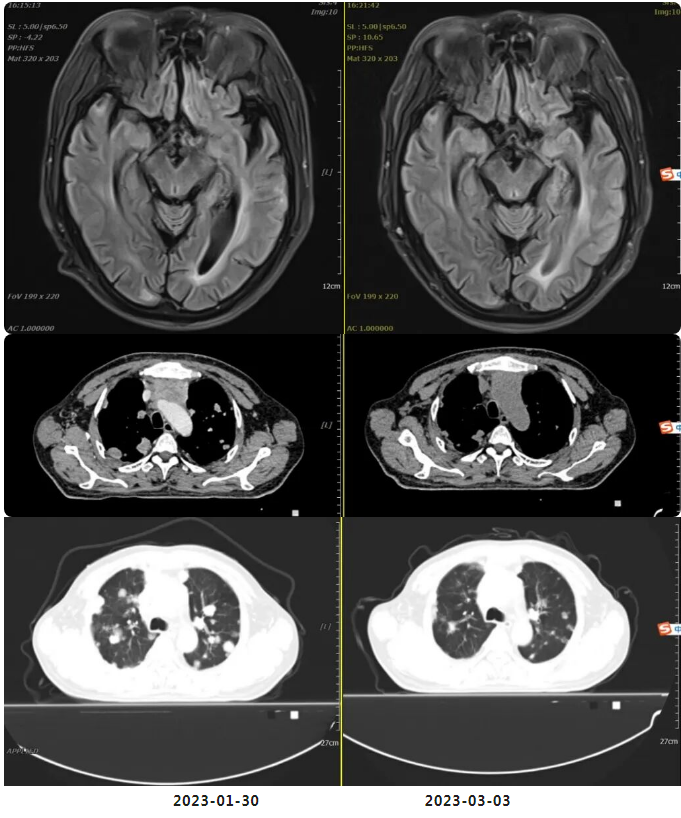
On January 30, 2023, initiated oral targeted therapy with Afatinib Maleate Tablets (patient-provided) at a dose of 40mg orally once daily.
On March 3, 2023, underwent follow-up non-contrast chest-abdomen-pelvis CT and non-contrast head MRI at our hospital. Efficacy evaluation: SD (Stable Disease).
Preliminary Review: Zhang Jie
Final Review: Ma Shuqian
