Clinical Observation of Abscopal Effect in Recurrent Thymic Carcinoma Treated with Carbon Ion Therap
Clinical Observation of Abscopal Effect in Recurrent Thymic Carcinoma Treated with Carbon Ion Therapy: A Case Report
Chinese Journal of Clinical Oncology 2021, Vol. 48, No. 20
Authors: Zhang Yihe, Zhang Yanshan, Li Xiaojun, Pan Xin
Affiliation: Heavy Ion Center, Wuwei Cancer Hospital (Wuwei City, Gansu Province, 733000)
Corresponding Author: Zhang Yanshan (Email: 13830510999@163.com)
Keywords: Carbon ion abscopal effect, Bystander effect, Thymic carcinoma
DOI: 10.12354/j.issn.1000-8179.2021.20211145
Case Report: A 43-year-old female patient with no significant past medical history, denies smoking or chronic lung disease. The patient presented to Wuwei Cancer Hospital in February 2009 with complaints of palpitations and shortness of breath for 4 months, 10 years following postoperative radiotherapy for thymic carcinoma. Chest CT revealed a mass in the anterior superior mediastinum, suggesting a thymic tumor with invasion of the pericardium and left lung (Figure 1A). On February 16, 2009, the patient underwent "extensive resection of thymic tumor + partial resection of the left upper lobe of the lung, phrenic nerve, and pericardium." Postoperative pathology showed: (anterior superior mediastinum) thymic non-keratinizing squamous cell carcinoma. The tumor invaded the pericardium and lung, with no involvement of major blood vessels or lymph nodes. Pathological diagnosis: Masaoka stage IIIa, type C: thymic carcinoma. Postoperative chest CT indicated: postoperative thymic carcinoma, triangular effusion under the left sternum and effusion in the anterior pericardial space, extensive thickening and adhesion of the left pleura with a small amount of pleural effusion, local bone defect after sternal surgery, and soft tissue swelling (Figure 1B). Postoperative diagnosis: pT3N0M0 stage IIIa, based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging. The patient received postoperative intensity-modulated radiotherapy (IMRT) 35 days after surgery, with a dose of 50 Gy delivered in 25 fractions, targeting the original tumor bed and the upper mediastinum. Thereafter, the patient received no further treatment and underwent regular chest CT follow-ups. In December 2019, the patient again experienced palpitations and shortness of breath. Chest CT revealed multiple nodules in the left pleura, diaphragmatic crura, and peritoneum, with the largest lesion (6.3 cm × 5.2 cm) located near the pericardium in the left pleura, as well as multiple small lymph nodes in the left neck root, mediastinum, and retroperitoneum, suggesting recurrence and metastasis of thymic carcinoma. The clinical diagnosis was rcT2N2M1a stage IVa. After multidisciplinary team (MDT) consultation, considering the patient’s long disease-free survival after the previous surgery and radiotherapy, and the tumor’s indolent biological behavior, palliative carbon ion radiotherapy was administered to the largest tumor near the left pericardium, which was compressing and invading the heart, to alleviate the patient’s symptoms of palpitations and shortness of breath. Carbon ion therapy was chosen due to its unique biological and physical advantages. The biological characteristics of carbon ions include higher linear energy transfer (LET) and greater relative biological effectiveness (RBE), as well as a low oxygen enhancement ratio (OER) that is unaffected by tumor hypoxia, thereby improving local tumor control. [1]The physical characteristics of carbon ions, such as the Bragg peak and minimal lateral scattering, allow for more precise radiation dose distribution1. Thus, while delivering a high dose to the tumor, the radiation dose to the heart and lungs can be minimized, protecting critical organs. Treatment plan: The patient was placed in the prone position with hands crossed above the head, left hand on top, and a pillow under the forehead. Dual immobilization was achieved using a large vacuum cushion and thermoplastic film. CT scans were performed with a slice thickness of 3 mm, including plain, contrast-enhanced, and 4D sequences. The CT images were transferred to the "rtStation" planning system for target delineation. Target delineation: The gross tumor volume (GTV) included the largest tumor visible on imaging near the left pericardium; the clinical target volume (CTV) was defined as GTV with a 5 mm margin; the planning target volume (PTV) was defined as CTV with a 5 mm margin. Adjustments were made to protect critical organs, with a 1 cm safety margin left near the left ventricle. Treatment planning: Carbon ion treatment planning was performed using the Ci-plan software. The treatment plan required that 90% of the PTV receive the prescribed dose. One left lateral field and one vertical field were used, with a total dose of 60 GyE delivered to the lesion (Figure 2). The single dose was 4 GyE, administered once daily, 5 times per week (Monday to Friday), for a total of 12 fractions over 3 weeks. The dose of carbon ions was expressed in photon equivalent dose (GyE), where GyE is defined as the physical dose multiplied by the RBE of carbon ions, with the with a relative biological effectiveness (RBE) of carbon ions typically set at 3.0[2]. During and after treatment, the patient received no other therapies. After treatment, the patient’s symptoms of palpitations and shortness of breath were relieved. During treatment, only mild skin pigmentation was observed at the treatment site, classified as grade 1 skin reaction according to the Radiation Therapy Oncology Group (RTOG) acute radiation injury grading criteria, and grade 1 radiation dermatitis according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The skin reaction resolved completely 2 months after radiotherapy. Chest CT performed on the day of treatment completion showed not only shrinkage of the largest lesion at the irradiated site (left pericardium), but also varying degrees of shrinkage in metastatic lesions at other sites that received low-dose or no irradiation (left chest wall and left diaphragmatic crus), with continued shrinkage observed during follow-up up to 1 year (Figures 3A–3D). The maximum diameters of the left pericardial lesion before treatment and at 1 year post-treatment were 6.3 cm, 4.6 cm, and 3.9 cm, respectively; the maximum diameters of the left chest wall lesion were 2.8 cm, 1.9 cm, and 0.8 cm, respectively; and the maximum diameters of the left diaphragmatic crus lesion were 4.1 cm, 3.9 cm, and 3.4 cm, respectively. According to the RECIST 1.1 criteria for solid tumor response evaluation, the treatment outcome was assessed as partial response (PR). Due to the unique physical advantages of carbon ions, the radiation dose to critical organs such as the heart and lungs was reduced, better protecting normal tissues. The patient experienced no acute radiation injuries of grade ≥2 during the entire treatment period, demonstrating the safety and efficacy of the treatment approach.
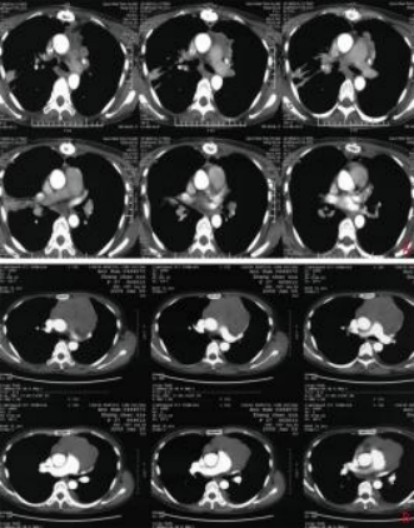
A: Preoperative chest contrast-enhanced CT (January 9, 2009); B: Postoperative chest contrast-enhanced CT (February 21, 2009)
Figure 1 Preoperative and postoperative imaging
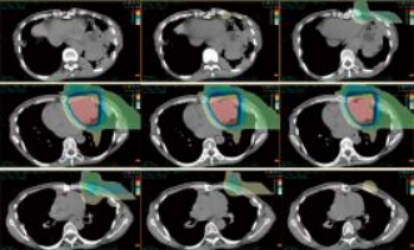
Figure 2 Beam direction and composite dose distribution (one left lateral field + one vertical field)
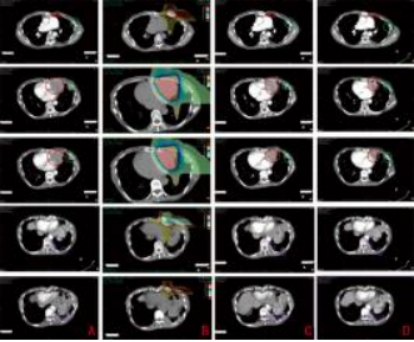
A: Before carbon ion therapy (March 24, 2020); B: Dose distribution map (1%–110%); C: After carbon ion therapy (April 10, 2020); D: One year after carbon ion therapy (April 19, 2021); Red - Target dose irradiation area; Green - Low-dose irradiation area; Purple - Non-irradiated area
Figure 3 Imaging of chest lesions before and after treatment
Summary
The abscopal effect refers to the phenomenon in which irradiation of a tumor target area induces shrinkage or even disappearance of tumors in non-irradiated regions. Its mechanism may involve local radiotherapy not only directly kills tumor cells but also promotes the release of pro-inflammatory factors by immune cells, which act on non-irradiated tumor sites, inducing chromosomal breaks or aberrations in tumor cells in non-irradiated areas, thereby causing tumor shrinkage or disappearance. Additionally, it may trigger a systemic anti-tumor immune response by releasing tumor-associated antigens and altering tumor cell phenotypes, thereby inhibiting the growth of tumors outside the irradiation field. The occurrence of the abscopal effect may be related to radiotherapy-induced in situ tumor vaccination. The in situ vaccine effect refers to the phenomenon where radiotherapy triggers acquired immune responses and specific antigen presentation reactions at the irradiated tumor site. Sharabi et al. demonstrated that a single high dose of radiotherapy enhances the presentation of tumor-associated antigens and improving T cell recognition, making it easier to induce the abscopal effect. This may be related to the sensitivity of different tumors to radiation, the characteristics of the radiation itself, the single radiation dose, and whether it is combined with other treatments. The abscopal effect has been observed in various tumors, especially in cases where conventional radiotherapy is combined with immune inhibitors, including malignant lymphoma, liver cancer, cervical cancer, melanoma, and colorectal cancer. However, there are few reported cases of the abscopal effect observed in patients with postoperative recurrent and metastatic thymic carcinoma after pure carbon ion radiotherapy and during follow-up. In this case, the abscopal effect induced by carbon ion therapy persisted until the last observation, one year after the end of treatment. The non-irradiated tumors shrank immediately after carbon ion therapy without any other treatment and continued to shrink during follow-up, as clearly observed on contrast-enhanced CT. Nevertheless, the mechanism of immune enhancement in non-irradiated tumors remains unclear. The combination of immune checkpoint inhibitors and radiotherapy has increased the incidence of the abscopal effect. However, in this case, the patient did not receive any immune or other drug treatments, and the abscopal effect was entirely induced by carbon ions. Whether the abscopal effect induced by pure carbon ion therapy is due to the tumor's inherent sensitivity to radiation, the patient's unique immune system, or the unique physical and biological advantages of carbon ions is difficult to determine. Whether this mechanism is related to the abscopal effect induced by photon irradiation and whether it can be further enhanced by combining it with immunotherapy requires further research.
Reference
[1]Masashi K, Yusuke D, Junichi S, et al. Multicenter study of carbon- ion radiation therapy for mucosal melanoma of the head and neck: subanalysis of the Japan carbon-ion radiation oncology study group (J-CROS) study (1402 HN)[J]. Int J Radiat Oncol Biol Phys, 2018, 102(2):353-361.
[2]Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HI-MAC clinical irradiation system for heavy-ion radiation therapy[J]. Int J Radiat Oncol Biol Phys, 1999, 44:201-210.
[3]Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation ther-apy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen[J]. Cancer Im- munol Res, 2015, 3(4):345-355.
Received: July 17, 2021
Edited by: Sun Xijia
Proofread by: Fan Juan
