Efficacy of Carbon Ion Therapy for Intracranial Metastasis of Glioblastoma After Surgery
Efficacy of Carbon Ion Therapy for Intracranial Metastasis of Glioblastoma After Surgery
Glioblastoma (GBM) is the most common primary malignant tumor of the central nervous system in adults, classified as a grade IV high-grade glioma by WHO. The biological characteristics of GBM include diffuse and infiltrative growth of tumor cells, rapid proliferation, difficulty in complete surgical resection, and tendency for in situ recurrence postoperatively. The current standard treatment regimen involves maximal safe tumor resection, combined with temozolomide (TMZ) adjuvant therapy and concurrent chemoradiotherapy with TMZ. Additionally, comprehensive treatment approaches such as immunotherapy, targeted therapy, and electric field therapy are also utilized. Radiotherapy can eradicate or inhibit tumor cells, prolonging patient survival, with conventional fractionated external beam radiation being the standard treatment for glioblastoma radiotherapy.
Overall, the prognosis of patients with this disease is poor, with a median survival of less than 2 years. Despite standard treatment, recurrence or metastasis can still occur, with approximately 5% of glioblastoma patients developing distant multifocal metastases after standard treatment. The reason for this is that the primary tumor is close to the cerebrospinal fluid system, and opening the cerebrospinal fluid system during surgery can significantly increase the probability of intracranial tumor dissemination. When recurrence occurs, treatment options are limited, including repeat surgery, re-irradiation, systemic chemotherapy, immunotherapy, molecular targeted therapy, TTF, and best supportive care. However, local treatment remains the most beneficial approach for patients, and whether carbon ion therapy offers advantages is explored in the following case study of a patient with glioblastoma who experienced local recurrence and intracranial metastasis after surgery.
Patient is a 58-year-old male presenting with complaints of glioma recurrence and metastasis one year after brain surgery.
The patient presented with expressive aphasia on August 1, 2022. An MRI of the brain in August 2022 revealed a left frontal lobe glioblastoma measuring 45x42x35mm. On August 25, 2022, the patient underwent a left frontal-temporal craniotomy for resection of the intracranial mass. Postoperative pathology results indicated a glioblastoma, NOS (CNS WHO grade IV). Immunohistochemical results showed: GFAP (+), Olig-2 (+), IDH1 (-), ATRX (+), p53 (scattered +), Ki-67 (approximately 40%), H3K27me3 (+); and another sample showed PHH3 (scattered +), Ki-67 (approximately 20%). Molecular pathology indicated that the MGMT promoter was unmethylated. The patient received 33 sessions of postoperative radiotherapy, concurrent with temozolomide at 100mg, followed by sequential treatment of 300mg for 12 sessions, which concluded in September of this year.
Physical examination: The patient is alert, with normal mental status, but has lost the ability to express and communicate. There is a significant decline in memory, orientation, comprehension, calculation, and judgment abilities upon rough assessment. There is no drooping of the eyelids, and the palpebral fissures are of equal size. No abnormal protrusion or retraction of the eyeballs is observed; the eye movements in all directions are intact. Both pupils are equal in size and round, measuring 2.5mm, with both direct and indirect light reflexes present. Accommodation and convergence reflexes show no abnormalities. Tongue extension shows no deviation, and there is no atrophy or tremor of the tongue muscles. Muscle strength is graded at 3/5 in the right upper and lower limbs, and 4/5 in the left limbs, with muscle atrophy noted in the right lower limb. Babinski sign: (-), Gordon sign: (-), Oppenheim sign: (-), Hoffmann sign: (-).
Carbon ion therapy plan: The prescribed dose for the cerebellum is 60Gy (RBE) in 20 fractions; the prescribed dose for the left frontal-temporal tumor is 45Gy (RBE) in 15 fractions. The concurrent chemotherapy regimen includes 2 cycles of EP regimen: Etoposide 150mg IV infusion on days 1-3; Cisplatin 40mg on days 1-3; every 3 weeks. Bevacizumab 700mg every 2 weeks. After treatment, follow-up showed that the cerebellar lesion reduced from 3.8cm x 3.4cm to 2.1cm x 1.5cm.
After treatment, the patient's expressive and communication abilities have improved. Memory and orientation have also improved. There is no drooping of the eyelids, and the palpebral fissures are of equal size. No abnormal protrusion or retraction of the eyeballs is observed; eye movements in all directions are good. Both pupils are equal in size and round, measuring 2.5mm, with both direct and consensual light reflexes present. Accommodation and convergence reflexes show no abnormalities. Tongue extension shows no deviation, and there is no atrophy or tremor of the tongue muscles. Muscle strength is graded at 3/5 in the right upper and lower limbs, while muscle strength in the left limbs has returned to normal. There is no muscle atrophy noted in the right lower limb. Babinski sign: (-), Gordon sign: (-), Oppenheim sign: (-), Hoffmann sign: (-).
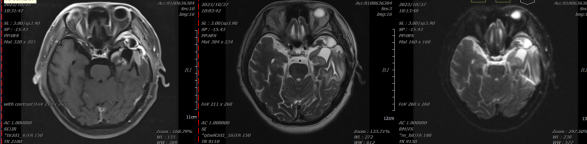
Figure 1: Before treatment, there is localized absence of the frontal, temporal, and parietal bones. Abnormal signals are observed in the left basal ganglia and left temporal lobe, which are mostly considered to indicate postoperative recurrence. Cystic lesions in the surgical area are mostly considered to be postoperative residual cavities with enhancement of the adjacent meninges in the surgical region.
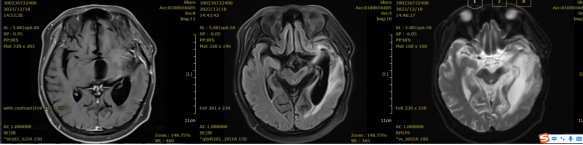
Figure 2: Comparison of the left basal ganglia and left temporal lobe before and after treatment. Irregular isointense T1 and mixed slightly long T2 signal shadows are visible in the left basal ganglia and left temporal lobe, surrounded by extensive edema signals, with no enhancement on contrast scan. There is localized absence of the frontal, temporal, and parietal bones. Abnormal signals in the left basal ganglia and left temporal lobe show a reduction in lesion extent and decreased enhancement compared to the pre-treatment condition.
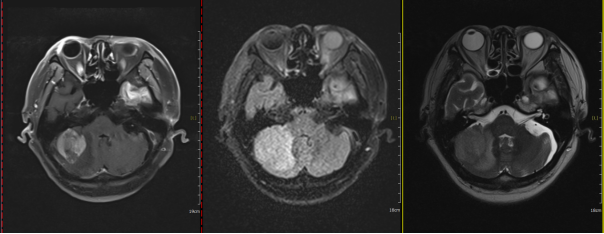
Figure 3: Before treatment, a space-occupying lesion in the right cerebellar hemisphere with an axial diameter of approximately 3.8 cm x 3.4 cm.
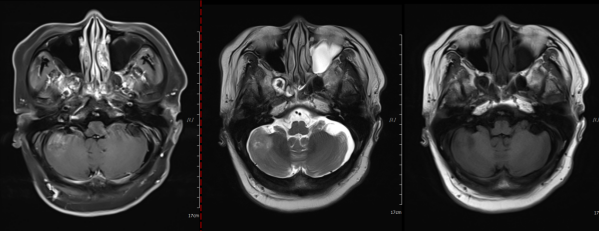
Figure 4: After treatment, a space-occupying lesion in the right cerebellar hemisphere with an axial diameter of approximately 2.1 cm x 1.7 cm.
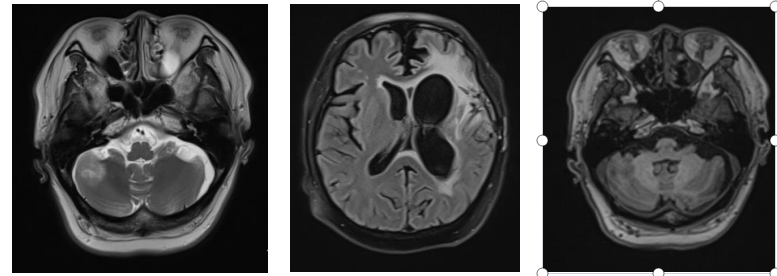
Figure 5: Three months after radiotherapy, the right cerebellar hemisphere shows a lesion with an axial diameter of approximately 2.1 cm x 1.5 cm.
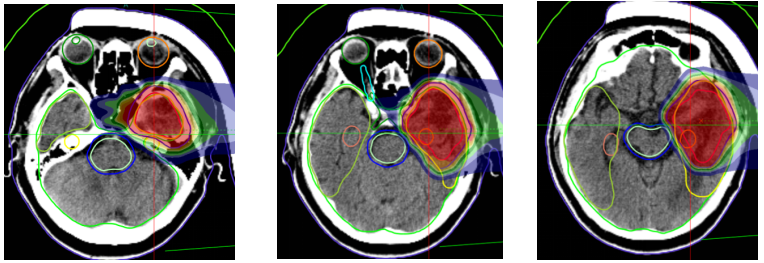
Figure 6: The prescribed dose for the left frontotemporal lobe mass is 45 Gy (RBE) in 15 fractions.
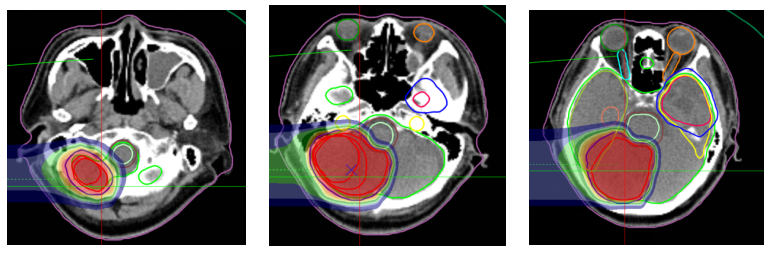
Figure 7: Treatment plan: Prescription dose: The PTVm prescription dose is 60 Gy (RBE) in 20 fractions.
Glioblastoma cells can disseminate through fiber tracts and the cerebrospinal fluid (CSF) system, and the tumor can spread via either of these pathways. Approximately 5% of glioblastoma patients develop distant multifocal metastases after standard treatment. However, the proximity of the primary tumor to the CSF system or the intraoperative opening of the CSF system can significantly increase the probability of intracranial tumor dissemination. For the treatment of recurrent and metastatic glioblastoma, options include surgery, stereotactic radiotherapy, and tumor-treating fields. Carbon ions possess unique physical properties and biological effects. Unlike conventional photon beams, heavy ions release less energy along the incident path but deposit a large amount of energy at the end, forming a Bragg peak. Beyond the Bragg peak, the exit path carries almost no effective dose. Additionally, carbon ion radiation is a type of high linear energy transfer (LET) radiation, which can directly cause DNA double-strand breaks in tumor cells, and its effectiveness is nearly unaffected by cell cycle phase or oxygen concentration, making it more effective in killing hypoxic tumor cells. The high biological effect of carbon ions is primarily confined to the Bragg peak region, concentrating the biological effect more precisely within the tumor area and avoiding high doses and damage to surrounding normal tissues.
Re-irradiation is another option considered as salvage therapy for recurrent glioblastoma (rGBM). However, re-irradiation is often controversial due to the risk of toxicity. In fact, high-dose radiation therapy (approximately 60 Gy) is typically used as first-line treatment to reduce the risk of in-field recurrence, which generally precludes the use of a second full-dose radiation course. Nevertheless, re-irradiation has been shown to be valuable for local recurrence. Newer treatment techniques, such as carbon ion radiation, enable more precise delivery of radiation to the target area without exceeding the tolerance of surrounding normal structures.
Radiotherapy Department Five: Hu Tingchao, Wang Xinlan, Zhang Yaoling, Ma Shuping, Zhang Tian'e, Tong Zongze.
