A Case Report on Combined Photon and Carbon Ion Therapy for Glioblastoma
A Case Report on Combined Photon and Carbon Ion Therapy for Glioblastoma
Patient Qu, a 61-year-old female, presented with progressive speech difficulties three months prior to admission. PET-CT revealed multiple irregularly shaped MET and FDG hypermetabolic lesions in the left frontal cortex, subcortical left frontal lobe adjacent to the left ventricle, the left optic tract region of the suprasellar cistern, and the sellar region. A biopsy confirmed the diagnosis of glioblastoma, WHO grade IV. She was admitted for carbon ion therapy. Upon admission, the patient exhibited speech difficulties, with largely intact comprehension, judgment, calculation, and memory, but slightly impaired orientation. Following treatment, her speech difficulties and orientation showed improvement.
Treatment plan: Combined photon and carbon ion therapy (CIRT):
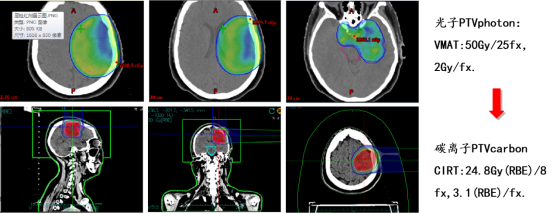
Efficacy comparison:
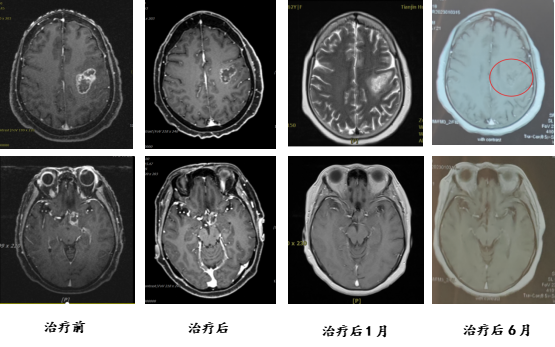
Discussion:
Glioma is currently the most common primary malignant tumor of the central nervous system in adults in China. According to the 2023 "China Cancer Development Report," the annual incidence of glioma is approximately 6.4 per 100,000 people. Nearly half of these cases are glioblastoma (GBM). The current standard treatment involves maximal safe surgical resection, followed by concurrent temozolomide chemoradiotherapy and adjuvant temozolomide therapy. Despite this standard postoperative photon chemoradiotherapy, the median overall survival for patients remains relatively short, at 14 to 17 months.
Carbon ion radiotherapy (CIRT) for the treatment of gliomas began in the 1990s. The advent of CIRT has provided a new option for patients with high-grade gliomas, particularly those with recurrent high-grade gliomas. A clinical study on CIRT boost for high-grade gliomas included 48 patients who underwent surgical treatment, nimustine chemotherapy, and conventional photon radiotherapy (50 Gy/25 fractions), followed by a CIRT boost. Based on the CIRT dose, patients were divided into three groups: low-dose (16.8 Gy (RBE)), medium-dose (18.4–22.4 Gy (RBE)), and high-dose (24.8 Gy (RBE)). The median overall survival (mOS) for the three groups was 7, 19, and 26 months, respectively, with no severe acute or chronic adverse effects reported. Univariate analysis showed that higher CIRT doses were associated with better outcomes.
As of August 2023, follow-up data from our center analyzed 34 treated high-grade glioma patients who underwent combined photon and carbon ion therapy. The longest follow-up period was 38 months, with a progression-free survival (PFS) of 15 months and a median overall survival (OS) of 28 months. The OS rates at 12 and 24 months were 90.0% and 57.9%, respectively. Compared to conventional photon radiotherapy in the Stupp study, the median PFS was nearly doubled, the median OS increased by 13.4 months, the 1-year survival rate improved by 28.9%, and the 2-year survival rate improved by 31.4%. These results far exceed the 1-year survival rate of 61.1% with conventional photon radiotherapy, and some high-grade glioma patients achieved survival periods exceeding 4 years.
Carbon ion beams have a finite range and a sharp high-dose Bragg peak. Among various ions, carbon ions are considered to have the most advantageous properties in terms of biological effectiveness and dose localization, making them the preferred choice for cancer treatment. Protons and photons have similar relative biological effectiveness (RBE), with a peak value of approximately 1.0–1.1. They primarily cause indirect ionization, generating free radicals that lead to DNA double-strand or single-strand breaks, resulting in lethal or sublethal damage. Protons and photons have limited ability to kill tumor cells in the S and G0 phases or under hypoxic conditions, and their effectiveness is highly dependent on the cell cycle. In contrast, the RBE of carbon ion radiotherapy (CIRT) in the Bragg peak region is 2.5–3.0. CIRT causes direct ionization, leading to extensive DNA double-strand breaks and lethal damage, effectively killing tumor cells. Additionally, the tumor-killing ability of carbon ions is less dependent on factors such as oxygen concentration, cell cycle distribution, and sublethal damage repair. The "4R theory" of traditional fractionated radiotherapy does not apply to carbon ions.
In vitro cellular studies on gliomas have shown that the relative biological effectiveness (RBE) of carbon ion therapy ranges from 2.10 to 3.44 on average. Due to the higher RBE of carbon ion radiation, its radiobiological advantages can translate into enhanced clinical efficacy. The combined application of photons and carbon ions in the treatment of gliomas not only improves tumor control rates but also reduces high-dose radiation damage to surrounding tissues, thereby decreasing the incidence of brain injury. This approach offers a novel therapeutic strategy for glioma radiotherapy.
Radiotherapy Department Five: Hu Tingchao, Wang Xinlan, Zhang Yaoling, Ma Shuping, Zhang Tian'e, Tong Zongze.
