Intraperitoneal Hyperthermic Perfusion Chemotherapy (HIPEC) – Heating Hope to 43°C
Intraperitoneal Hyperthermic Perfusion Chemotherapy (HIPEC) – Heating Hope to 43°C
Intraperitoneal Hyperthermic Perfusion Chemotherapy (HIPEC) – Offering a chance for reversal to patients with peritoneal tumors.
Peritoneal metastasis was once considered the "terminal stage" of gastrointestinal cancers. However, with continuous advancements in medical technology, a new treatment modality that is precise, effective, and relatively gentle – Intraperitoneal Hyperthermic Perfusion Chemotherapy (HIPEC) – is bringing hope to a growing number of patients, allowing them to see their lives "brighten" again.
Why is HIPEC performed?
Conventional surgery can remove visible tumor lesions and adjacent tissues but cannot completely eliminate subclinical lesions and free cancer cells that are invisible to the naked eye. Residual cancer cells can lead to tumor implantation, metastasis, recurrence, and even cause malignant pleural or peritoneal effusions.
Hyperthermic perfusion chemotherapy can fill and bathe the entire body cavity with perfusion fluid, directly destroying microscopic lesions smaller than 5mm and free cancer cells. This approach can effectively improve survival rates, enhance quality of life, and reduce postoperative recurrence rates.
What is HIPEC? In one sentence:
It involves continuously circulating and perfusing chemotherapy drugs heated to 42–43°C within the abdominal cavity, allowing the combined effects of heat and high drug concentrations to precisely kill tumor cells.
It is not merely hyperthermia, nor is it standard chemotherapy. Instead, it represents the synergy of three tumor-killing mechanisms: Hyperthermia + High-concentration Chemotherapy + Intraperitoneal Circulating Perfusion.
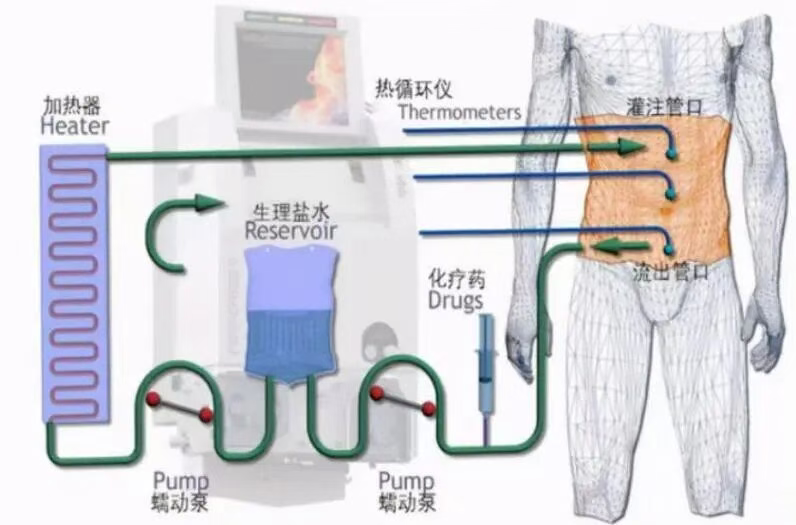
Why can HIPEC kill tumors? – Detailed explanation of the mechanisms
1. Hyperthermia-induced Cell Kill (Hyperthermia) – The "selective heat strike" at 42–43°C High temperature targets inherent weaknesses of tumor cells: Tumor cells are more sensitive to heat. Heat can damage the protein structure of tumor cells. Heat can inhibit DNA repair in tumor cells. High temperature can induce a "heat shock response" in cancer cells, leading to apoptosis or necrosis, while normal tissues are relatively tolerant to 42°C. Therefore, hyperthermia can selectively destroy tumor cells.
2. High-Concentration Local Chemotherapy (High Concentration) The drug concentration within the peritoneal cavity can be 20–100 times higher than that achieved with intravenous chemotherapy! The drugs act directly on peritoneal cancer cells and microscopic lesions, resulting in strong efficacy and broad coverage, while systemic toxicity is lower.
3. Intraperitoneal Circulating Perfusion (Perfusion) The chemotherapeutic solution is continuously circulated within the abdominal cavity, providing comprehensive irrigation to the peritoneal mesentery, pelvis, subphrenic space, and residual tumor sites. This process helps to clear free cancer cells, invisible microscopic implants, clusters of tumor cells floating in the peritoneal cavity, thereby reducing peritoneal recurrence and controlling ascites.
How is HIPEC performed?
HIPEC can be performed during surgery, via minimally invasive laparoscopy, or as a bedside procedure (primarily for ascites control).
1. A multidisciplinary team (including surgery, medical oncology, anesthesiology, ICU, radiology, pathology) assesses suitability for HIPEC based on: Tumor type Peritoneal Carcinomatosis Index (PCI) Overall patient condition Presence of distant metastases Cardiopulmonary fitness to tolerate the procedure Potential for benefit.
2. Proceed to the operating room/laparoscopy suite. If necessary, Cytoreductive Surgery (CRS) is performed first, which may involve: Abdominal exploration Removal of resectable tumor nodules (CRS) Relief of obstructions, management of adhesions Preparation for perfusion.
3. Placement of HIPEC catheters and temperature monitoring probes. Typically placed within the abdomen are: Inflow catheter(s) Outflow catheter(s) Multiple temperature probes This ensures even circulation and consistent temperature of the perfusate.
4. Preparation and heating of the chemotherapeutic drugs. The chemotherapeutic solution is precisely heated to 42–43°C and maintained at a constant temperature using a HIPEC machine. Commonly used drugs include: Oxaliplatin, Cisplatin, Doxorubicin, Paclitaxel (tailored to the tumor type).
5. Intraperitoneal perfusion and circulation for 60–90 minutes (Core step). The heated chemotherapeutic solution is circulated through the abdomen at a set rate, covering all areas. The machine continuously monitors: temperature, flow rate, return volume, and safety parameters. Nurses and doctors monitor the procedure throughout.
6. Rinsing, conclusion of perfusion, and abdominal closure. After perfusion, the abdomen is rinsed with a solution at normal or warm temperature. The incisions are then closed, and the patient is transferred to the recovery room or ICU for monitoring.

How is HIPEC performed?
① Control and alleviate cancerous ascites
Many patients experience significant reduction or long-term stabilization of ascites after HIPEC.
② Eliminate microscopic lesions in the abdominal cavity
Disrupt the chain of peritoneal recurrence.
③ Collaborate with surgery to achieve long-term survival benefits
In patients with peritoneal metastases from gastric cancer, colorectal cancer, ovarian cancer, etc., some can achieve significant extension of survival.
④ Alleviate symptoms and improve quality of life
- Reduced abdominal distension
- Improved appetite
- Easier mobility
- Better sleep
- Which patients are suitable for HIPEC?
- Gastric cancer Peritoneal metastasis High-risk post-operation (serosal invasion, poor differentiation, multiple positive lymph nodes)
- Colorectal cancer Peritoneal implantation metastasis Clear the abdominal cavity after relief of intestinal obstruction
- Ovarian cancer Initial treatment stage and recurrent stage Extensive residual abdominal lesions after surgery
- Primary peritoneal cancer / Pseudomyxoma peritonei
- Patients with massive and recurrent cancerous ascites
- Advantages of HIPEC in Our Department
- Multidisciplinary (MDT) joint decision-making
- Standardized HIPEC procedures and quality control
- Possession of advanced constant-temperature circulating perfusion equipment
- Surgical team with extensive practical experience in CRS + HIPEC
- Individualized and precise chemotherapy medication
- Integration with the ERAS accelerated recovery system
- Emphasis on perioperative safety management
- Multiple successful cases of ascites improvement and symptom relief
Minimally Invasive Intervention Department
The Minimally Invasive Intervention Department at the Lanzhou Heavy Ion Center of Gansu Wuwei Cancer Hospital is led by President Wang Junjie and has been established by appointing renowned minimally invasive cancer intervention therapy experts from within and outside the province. Characterized by its focus on minimally invasive interventional therapies for tumors, the department complements radiotherapy, forming a comprehensive cancer treatment system.

The department is equipped with 30 beds, offering an excellent inpatient environment and complete facilities. It is outfitted with advanced medical equipment including a US-made GE digital subtraction angiography (DSA) machine, a large-bore spiral CT scanner, microwave radiofrequency thermal coagulation machines, pulsed radiofrequency machines, radioactive seed implantation and planning systems, high-definition ultrasound machines, and regional circulatory hyperthermic perfusion therapy machines. The department performs various minimally invasive procedures under DSA, CT, and ultrasound guidance. It also conducts minimally invasive treatments for common peripheral vascular diseases such as great saphenous vein varicosity, arteriovenous fistula, and diabetic foot.


Representative Technologies of the Department
- Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for abdominal cancers; Hyperthermic Intrathoracic Chemotherapy (HITHOC) for thoracic cancers.
- Radioactive Seed Implantation.
- CT-guided Ablation for Liver Tumors.
- CT-guided Microwave Thermal Coagulation for Pulmonary Nodules.
- CT-guided Argon-Helium Cryoablation for Pulmonary Nodules.
- Ultrasound-guided Microwave Thermal Coagulation for Liver Nodules.
- Ultrasound-guided Biopsy for Thyroid Nodules.
- Ultrasound-guided Biopsy for Axillary Lymph Nodes.
- Ultrasound-guided Microwave Thermal Coagulation for Thyroid Nodules.
- DSA-guided Deep Vein Thrombectomy.
- DSA-guided Pulmonary Artery Embolization.
- DSA-guided Transarterial Chemoembolization for Tumors.
- DSA-guided Peripheral Angioplasty.
- DSA-guided Aneurysm Embolization.
- Microwave Thermal Coagulation for Great Saphenous Vein Varicosity.
- DSA-guided Revision of Arteriovenous Fistula.
- DSA-guided Cerebral Angiography and Embolization.
- DSA-guided Transjugular Intrahepatic Portosystemic Shunt (TIPS)
