LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma...
LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma by interacting with HNRNPC to enhance the stability of ZEB1 and ZEB2 mRNAs
Biochemical and Biophysical Research Communications
Yanshan Zhang a, Weizuo Chen a, *, Tingting Pan a, Huijuan Wang b, Yinguo Zhang c, Chao Li c
a Department of Radiotherapy, Tumor Hospital of Wuwei, Wuwei, Gansu, 733000, China
b Department of Tumor Chemotherapy, Tumor Hospital of Wuwei, Wuwei, Gansu, 733000, China
c Department of Thoracic Surgery, Tumor Hospital of Wuwei, Wuwei, Gansu, 733000, China
ABSTRACT
Long non-coding RNAs (lncRNAs) are a group of transcripts, which can regulate the progression of esophageal squamous cell carcinoma (ESCC). According to the data of TCGA, Ladybird homeobox 2 antisense RNA 1 (LBX2-AS1) is a highly expressed lncRNA in ESCC samples. Herein, we chose it for further study. Furtherly, dysregulation of LBX2-AS1 was identified in ESCC tissues with metastasis. Loss-of function assays were conducted and revealed that LBX2-AS1 knockdown suppressed ESCC cell migration and epithelial-mesenchymal transition (EMT). Zinc finger E-box binding homeobox 1 (ZEB1) and zinc finger E-box binding homeobox 2 (ZEB2) are two EMT-related transcription factors. Since LBX2-AS1 promoted the EMT progress and simultaneously enhanced the level of ZEB1 and ZEB2, we further investigated whether LBX2-AS1 promoted cell migration and EMT in ESCC by regulating ZEB1 and ZEB2. Mechanism investigations revealed that RNA binding protein heterogeneous nuclear ribonucleoprotein C (HNRNPC) could interact with LBX2-AS1, ZEB1 and ZEB2, simultaneously. The similar function of HNRNPC in regulating migration and EMT process was demonstrated. ZEB1 has been reported as a positive transcriptional regulator of lncRNA. Therefore, further mechanism analysis was made to demonstrate whether ZEB1 could regulate the transcription of LBX2-AS1. Collectively, our data showed that ZEB1-induced upregulation of LBX2-AS1 promoted cell migration and EMT process in ESCC via enhancing the stability of ZEB1 and ZEB2.
1.Introduction
Esophageal cancer (EC) ranks sixth among all cancer-related death all over the world. Adenocarcinoma (AC) and squamous cell carcinoma (SCC) are two main histological subtypes of EC, among which, esophageal squamous cell carcinoma (ESCC) is the most predominant in China [1,2]. Despite the progress in therapeutic methods such as surgery, chemotherapy and radiotherapy, the prognosis of patients with ESCC remains unsatisfactory [3,4]. Hence, exploring the underlying molecular targets is essential for the treatment of ESCC.
Long non-coding RNAs (lncRNAs) are regarded as a class of non-coding transcripts which are longer than 200 nucleotides and lack of protein-coding ability [5]. Functionally, lncRNAs can regulate various complex biological processes via diverse mechanisms [6e8]. Some reports unraveled the regulatory role of lncRNAs in the migration and EMT process of ESCC [9e11]. In present study, we focused on exploring a novel functional lncRNA in ESCC. LBX2-AS1 was chosen from TCGA database due to its differential expression in ESCC samples. Its expression level in different ESCC patient samples was analyzed by qRT-PCR analysis. The high level of LBX2-AS1 in metastatic samples prompted us to determine the role of LBX2-AS1 in regulating cell migration and EMT process. Mechanistically, lncRNAs can exert functions in malignant tumors by interacting with RNA binding proteins (RBPs) to regulate the stability of their target mRNAs. For examples, long non-coding RNA growth-arrested DNA damage-inducible gene 7 (gadd7) modulated the mRNA stability of Cdk6 by interacting with TDP-43 [12]; lncRNA MACC1-AS1 promoted the mRNA stability of MACC1 via AMPK/Lin28 axis in gastric cancer [13]; long non-coding RNA colon carcinoma-1 (OCC-1) inhibited colorectal cancer cell growth by reducing the level of HuR protein and regulating targeted mRNAs [14]. In present study, we investigated that LBX2-AS1 regulated cell migration and EMT progress in ESCC by regulating the stability of ZEB1 and ZEB2 mRNAs. Mechanistically, ZEB1 can transcriptionally activate lncRNA [15,16]. Therefore, we detected the role of ZEB1 in regulating LBX2-AS1 transcription. In summary, our present study revealed the novel mechanism of LBX2-AS1 in the progression of ESCC.
2. Materials and methods
2.1. Clinical specimens
82 pairs of ESCC tissues and adjacent normal tissues were collected from patients who were diagnosed with ESCC at Tumor Hospital of Wuwei. Tissues excised during the esophagectomy were instantly frozen in liquid nitrogen and stored at -80°C until use. Patients enrolled in this study did not receive local or systemic treatment. The written informed consents had been obtained from participants. This study endowed the approval of the Ethic Committee of the Hospital.
2.2. Cell culture
ESCC cell lines (KYSE150, KYSE-410, KYSE450, EC109, EC9706 and TE-13) and the human normal esophageal epithelial cell line (HEEC) used in this study were purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA). All cell lines grew routinely in Roswell Park Memorial Institute-1640 medium (RPMI; Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen). Cells were cultured in a humidified incubator at 37°C with 5% CO2. The culture medium was replaced every three days. Cells were passaged as soon as cell attachment rate reached 80-90%.
2.3. qRT-PCR analysis
Total RNA was extracted from KYSE150 and EC109 cell lines by TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) following the standard method. RNA from each group was subjected to cDNA synthesis. The cDNA was treated with qPCR using One Step SYBR® PrimeScript™ RT-PCR Kit (Perfect Real Time; Takara, Tokyo, Japan) on Bio-Rad Real-Time PCR System (Bio-Rad, Hercules, CA, USA). The PCR amplification was conducted with the parameters as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 5 s and 61°C for 30 s. Comparative quantification was determined using the 2-ΔΔCt method. GAPDH was considered as the internal control.
2.4. Cell transfection
KYSE150 and EC109 cell lines were placed into six-well plates until cell confluence reached about 70%. Short hairpin RNAs (shRNAs) targeting LBX2-AS1 (sh-LBX2-AS1#1 and sh-LBX2-AS1#2), HNRNPC (sh-HNRNPC#1 and sh-HNRNPC#2), ZEB1 (sh-ZEB1#1 and sh-ZEB1#2) and ZEB2 (sh-ZEB2#1 and sh-ZEB2#2) were synthesized for RNA knockdown. Nonspecific shRNA was utilized as negative control (sh-NC). LBX2-AS1 or ZEB1 was overexpressed in cells by transfecting with pcDNA3.1 vector containing the whole sequence of LBX2-AS1 or ZEB1. Cells transfected with empty pcDNA3.1 vector were identified as the control group. Cells were transfected with indicated plasmids using Lipofectamine2000 transfection kit (Invitrogen, Carlsbad, CA, USA). 48 h post-transfection, cells were reaped for subsequent analysis.
2.5. Transwell of cell migration assay
The transfected KYSE150 and EC109 cell lines were rinsed with phosphate-buffered saline (PBS) and re-suspended in the serum-free culture medium. 200 μl of cell suspension (a total of 5 × 104 cells) was placed to the upper chamber of 24-well Boyden chambers (pore size: 8 μm, BD Biosciences, Franklin Lakes, NJ, USA). The lower chamber was filled with cultured medium with 20% FBS. The non-migrating cells were removed from the upper chamber using cotton swabs, whereas the cells migrating to the lower chamber were fixed with methanol and stained with crystal violet solution. At length, the stained cells were photographed under the inverted confocal microscope (magnification, ×100; Olympus Corporation, Tokyo, Japan), followed by quantitation from five random fields of view.
2.6. Western blot
Total protein was isolated from KYSE150 and EC109 cell lines, quantitated and diluted in loading buffer to the same concentration. After denaturation at 95°C, protein specimens were separated by electrophoresis on 10% SDS-PAGE and transferred onto Hybond membrane (Amersham, Munich, Germany). Thereafter, samples were sealed in 5% skimmed milk at room temperature for 2 h and cultured with primary antibodies (Abcam, Cambridge, UK) at 4°C all night. After thrice washing with Tris-buffered saline (TBST), membranes were cultured with secondary antibodies conjugated with horseradish peroxidase and thrice washed with TBST. Finally, signals of protein bands were visualized using enhanced chemiluminescence reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
2.7. Immunofluorescence assay
KYSE150 and EC109 cell lines were placed on culture slides for one day until adhered to the slides. Cells were thrice rinsed in PBS and fixed in ice-cold methanol-acetone for 10 min. Followed by blocking in 5% BSA for 10 min, cells were cultured with the primary antibodies in PBS for 2 h at room temperature. Afterwards, cells were rinsed three times in PBS and treated with the secondary antibodies for an hour. At last, the slides were dyed with 4-,6-diamidino-2-phenylindole (DAPI) for 10 min and analyzed by an Olympus confocal imaging system (Olympus, Tokyo, Japan).
2.8. Bioinformatics analysis
The expression profile of LBX2-AS1, ZEB1 and ZEB2 in esophagus cancer were acquired from TCGA dataset (http://gepia.cancer-pku.cn/index.html). The binding motif and predicted sequences of ZEB1 in LBX2-AS1 promoter region were obtained from JASPAR tool (http://jaspar.genereg.net/).
2.9. RNA immunoprecipitation (RIP) assay
RIP assay was carried out using Magna RIP™ RNA Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA, USA). KYSE150 and EC109 cells were lysed in RIPA buffer containing protease inhibitor cocktail and RNase inhibitor. Lysates were cultured with RIP buffer containing magnetic bead conjugated with human Ago2 antibody (Millipore) or mouse immunoglobulin G control (IgG). After digesting proteins with proteinase K, the immunoprecipitated RNA were acquired and purified. At last, the recovered RNA were analyzed by qRT-PCR.
2.10. Luciferase reporter assay
KYSE150 and EC109 cell lines were planted in 96-well plates at a density of 5000 cells per well. After incubation for one day, cells were transfected with the complex containing 5 ng of Renilla luciferase reporter and 50 ng of the firefly luciferase reporter. The relative luciferase activity of RNA was evaluated using dual luciferase reporter assay system (Promega Corporation, Madison, WI, USA).
2.11. Chromatin immunoprecipitation (ChIP) assay
The cross-linked KYSE150 and EC109 cell lines were cultured in 4% formaldehyde at room temperature and rinsed in PBS. Cell nuclei extracted from lysis buffer (50 mM HEPES, pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100) was rinsed in a mixture of 10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 1 mM EDTA. After re-suspending in the lysis buffer containing 10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.1% sodium deoxycholate, cell nucleus was sonicated into 200- to 1000-bp fragments and cultured with 10 mg anti-ZEB1 antibody bound-protein G beads (Life Technologies, CA, USA) overnight at 4 °C. Followed by purifying, qRT-PCR was applied to assess the relative enrichment of immunoprecipitated DNA.
2.12. Statistical analysis
Statistical analyses were performed Student's t-test and one-way ANOVA using GraphPad Prism 6.0 (GraphPad, San Diego, CA, USA) and SPSS 19.0 statistical software (SPSS, Chicago, IL, USA). Experimental data were expressed as the mean ± standard deviation (SD). P < 0.05 indicates data are considered statistically significant. Pearson correlation analysis was applied to assess the expression correlation.
3. Results
3.1. High level of LBX2-AS1 positively regulated the migratory ability of ESCC cells
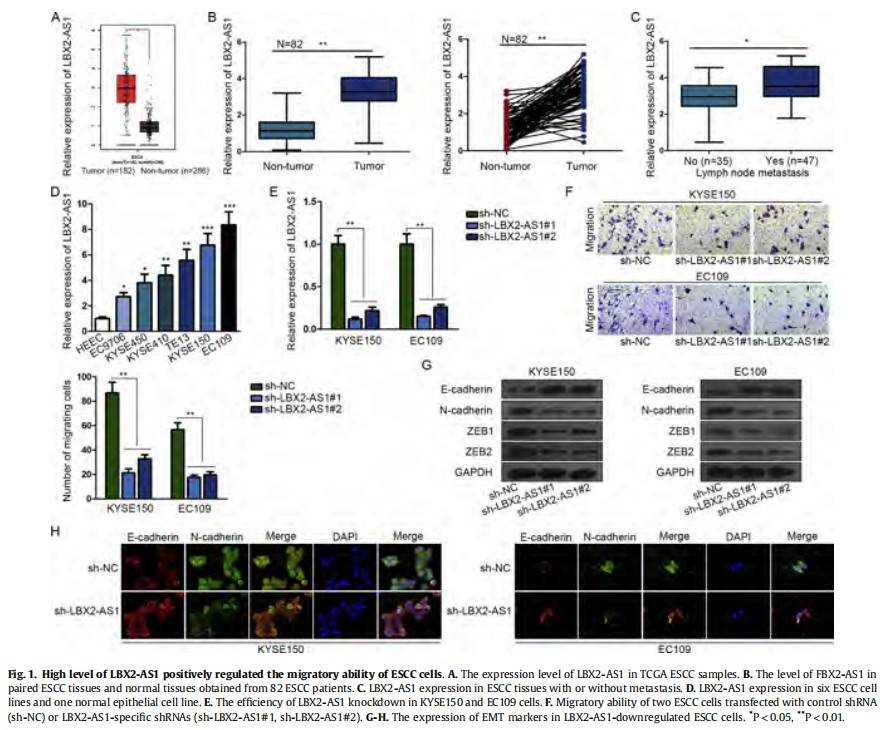
Searching from TCGA database, we found that LBX2-AS1 was significantly highly expressed in ESCC samples (Fig. 1A). High level of LBX2-AS1 was further validated in 82 ESCC tissues (Fig. 1B). To determine the potential biological role of LBX2-AS1 in ESCC, we examined its level in different ESCC tissues samples. qRT-PCR examination revealed that high level of LBX2-AS1 was associated with ESCC metastasis (Fig. 1C). Therefore, we hypothesized that LBX2-AS1 may be a regulator for ESCC cell migration. Before functional assays, we determined the level of LBX2-AS1 in six ESCC cell lines and a normal cell line. The highest level of LBX2-AS1 was detected in KYSE150 and EC109 cells (Fig. 1D). Thus, we performed loss-of function assays in above two cell lines. The knockdown efficiency was determined and shown in Fig. 1E. Functionally, knockdown of LBX2-AS1 efficiently suppressed cell migration (Fig. 1F). EMT process was examined in LBX2-AS1-downregulated ESCC cells. As illustrated in Fig. 1G, the protein level of E-cadherin (epithelial marker) was increased, while the protein levels of N-cadherin (mesenchymal marker) and EMT-related transcription factors (ZEB1 and ZEB2) were decreased. Immunofluorescence assay also revealed the same experimental results to western blot (Fig. 1H).
3.2. LBX2-AS1 interacted with the RNA-binding protein HNRNPC
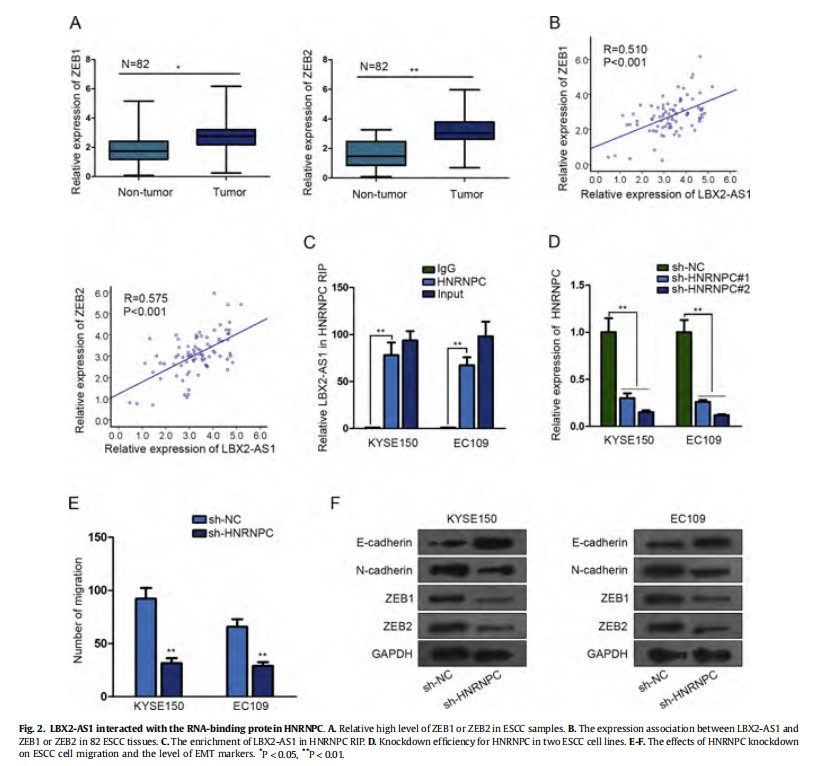
ZEB1 and ZEB2 are essential regulators in cell migration and EMT process. Since they were positively regulated by LBX2-AS1 in ESCC cells, we investigated whether LBX2-AS1 promoted cell migration and EMT process in ESCC by regulating ZEB1 and ZEB2. At first, we determined the high level of ZEB1 and ZEB2 in ESCC samples (Fig. 2A). Accordingly, the expression correlation between LBX2-AS1 and ZEB1 or ZEB2 was found to be positive (Fig. 2B). Previous reports revealed that lncRNAs can interact with RNA-binding proteins (RBPs) to regulate mRNA stability. In this regard, we explored whether LBX2-AS1 upregulated ZEB1 and ZEB2 by interacting with a RBP. Searching from Starbase (http://starbase.sysu.edu.cn/), HNRNPC can bind with LBX2-AS1, ZEB1 and ZEB2.RIP assay demonstrated the interaction between LBX2-AS1 and HNRNPC in ESCC cells (Fig. 2C). Functionally, knockdown of HNRNPC (Fig. 2D) efficiently suppressed cell migration and reversed EMT progress (Fig. 2EeF). These data indicated that LBX2-AS1 cooperated with HNRNPC to promote ESCC cell migration and EMT process.
3.3. LBX2-AS1 interacted with HNRNPC to increase the mRNA stability of ZEB1/2
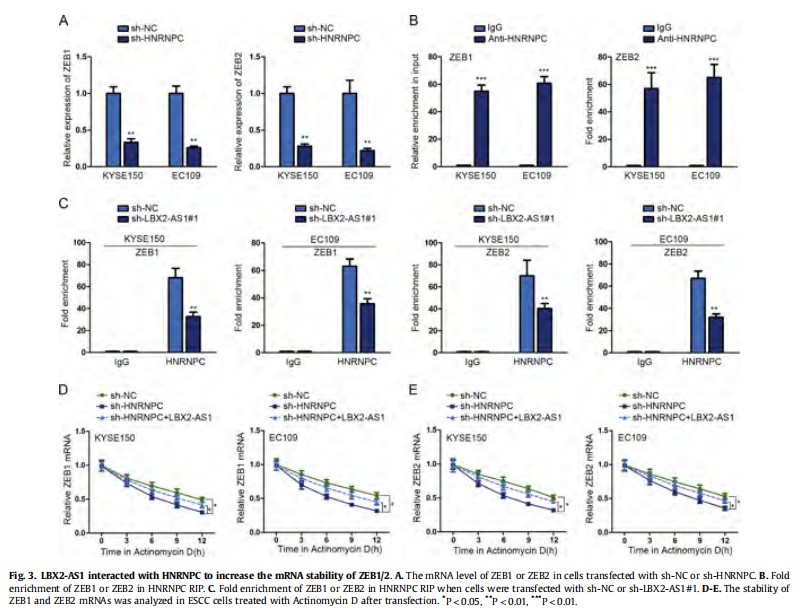
The potential interaction between HNRNPC and ZEB1/2 was predicted by using bioinformatics analysis. In this step, we performed mechanism experiments to make confirmation. The mRNA level of ZEB1 and ZEB2 was found to be positively regulated by HNRNPC (Fig. 3A). Similarly, RIP assay demonstrated that both ZEB1 and ZEB2 could bind with HNRNPC protein (Fig. 3B). Whereas, the binding capacity of ZEB1 and ZEB2 to HNRNPC protein was weakened by the knockdown of LBX2-AS1 (Fig. 3C), indicating that ZEB1 and ZEB2 were potential downstream mRNAs of LBX2-AS1 and HNRNPC in ESCC. Actinomycin D is known as an inhibitor of mRNA stability [17]. Therefore, we treated KYSE150 and EC109 cells with Actinomycin D to measure the mRNA stability of ZEB1 and ZEB2. The results indicated that knockdown of HNRNPC promoted the decay of ZEB1 and ZEB2 mRNAs (Fig. 3DeE). Whereas, the effects were attenuated by overexpression of LBX2-AS1. These results prompted us to conclude that LBX2-AS1-HNRNPC enhanced the stability of ZEB1 and ZEB2 mRNAs.
3.4. ZEB1 transcriptionally activated LBX2-AS1 in ESCC
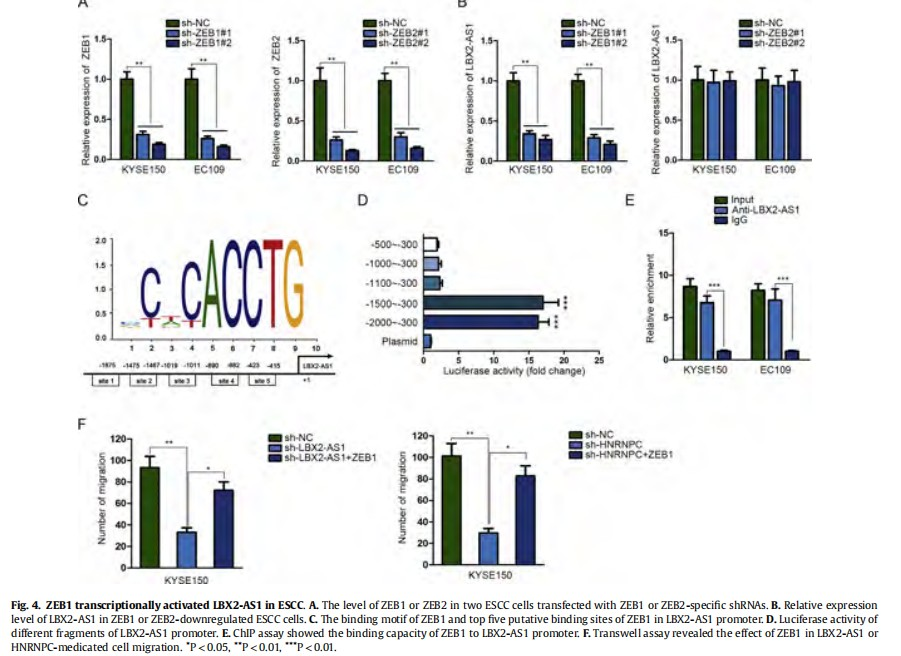
ZEB1 and ZEB2 can act as a transcriptional activator to regulate gene expression. In this study, we investigated whether ZEB1 and ZEB2 can regulate the LBX2-AS1 transcription, thereby forming a positive feedback loop in ESCC. At first, we treated two ESCC cells with ZEB1 or ZEB2-specific shRNAs (Fig. 4A) and examined the relative expression level of LBX2-AS1. As presented in Fig. 4B, the expression level of LBX2-AS1 was positively regulated by ZEB1 but not ZEB2. The binding motif of ZEB1 and top five putative binding sites of ZEB1 in LBX2-AS1 promoter were predicted from JASPAR (http://jaspar.genereg.net/) (Fig. 4C). Luciferase reporter assay performed in 293T cell demonstrated the fragment from -2000 to -1500 was responsible for the interaction between ZEB1 and LBX2-AS1 promoter (Fig. 4D). This interaction was further validated by ChIP assay (Fig. 4E). Thus, we confirmed that ZEB1 contributed to the transcriptional activation of LBX2-AS1 in ESCC. Finally, transwell assays revealed that overexpression of ZEB1 rescued the decreased cell migration induced by the knockdown of LBX2-AS1 or HNRNPC (Fig. 4F). Taken together, ZEB1 and LBX2-AS1 can form a positive feedback loop to regulate ESCC cell migration.
4. Discussion
LncRNAs can regulate the malignant progression of ESCC by acting as the biological processes [18-23]. To date, the novel molecular mechanism of lncRNAs still needs to be investigated. In current study, a differentially expressed lncRNA in ESCC samples was searched from TCGA database. LncRNA LBX2-AS1 has not been reported in ESCC. High level of LBX2-AS1 was closely correlated with the ESCC metastasis. Therefore, we hypothesized LBX2-AS1 might regulate ESCC cell migration or EMT. Furthermore, relative high expression level of LBX2-AS1 was determined in ESCC tissues with metastasis. The results indicated the potential involvement of LBX2-AS1 in ESCC migration. Functionally, knockdown of LBX2-AS1 inhibited cell migration, invasion and reversed EMT process. Therefore, we determined the role of LBX2-AS1 in promoting ESCC cell migration and EMT.
ZEB1 and ZEB2 are core factors correlated with the migration and EMT [24-27]. According to our experimental data, LBX2-AS1 can upregulate ZEB1 and ZEB2. Thus, we explored whether LBX2-AS1 promoted cell migration and EMT by upregulating ZEB1 and ZEB2 in ESCC.
Mechanistically, lncRNAs can act as regulators in human cancers by interacting with RBPs to regulate their downstream mRNAs [28-30]. Using bioinformatics analysis, HNRNPC was found to be a RBP which may bind to LBX2-AS1, ZEB1 and ZEB2. RIP assay demonstrated the interaction between HNRNPC protein and LBX2-AS1. Functionally, knockdown of HNRNPC suppressed cell migration and reversed EMT progress efficiently. Likewise, we determined the interaction between HNRNPC and ZEB1/2 in ESCC cells. Further rescue assays demonstrated that LBX2-AS1 affected the interaction between HNRNPC and ZEB1/2. More importantly, stability of ZEB1 and ZEB2 mRNAs was positively regulated by LBX2-AS1 and HNRNPC. These experimental data indicated that LBX2-AS1 stabilized ZEB1 and ZEB2 in ESCC by interacting with HNRNPC.
ZEB1 and ZEB2 are acknowledged as two transcription factors [31,32]. In our current study, we investigated whether they can regulate the LBX2-AS1 transcription. The expression level of LBX2-AS1 was found to be positively regulated by ZEB1 bout not ZEB2. Therefore, we further investigated whether ZEB1 can transcriptionally activate LBX2-AS1 in ESCC. According to mechanism experiments, we determined the positive effect of ZEB1 on the transcription of LBX2-AS1. Therefore, we concluded that LBX2-AS1 was activated by ZEB1 and interacted with HNRNPC to stabilize ZEB1 and ZEB2 mRNAs. Finally, we conducted rescue assays in ESCC cell lines to validate the role of ZEB1 in LBX2-AS1 or HNRNPC-mediated cell migration. The experimental data demonstrated that the migratory ability of ESCC cells decreased by the knockdown of LBX2-AS1 or HNRNPC was recovered by overexpression of ZEB1. Therefore, we concluded that ZEB1-induced upregulation of LBX2-AS1 promoted cell migration and EMT in ESCC by interacting with HNRNPC to stabilize ZEB1 and ZEB1 mRNAs. Our research findings revealed a novel mechanism of lncRNA LBX2-AS1 and advanced our understanding of lncRNA-regulated mechanism in the progression of ESCC.
Disclosure of interests
None.
Acknowledgement
The authors sincerely appreciate all members participated in this study.
Transparency document
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrc.2019.02.079.
References
[1] H. Zeng, R. Zheng, S. Zhang, T. Zuo, C. Xia, X. Zou, W. Chen, Esophageal cancer statistics in China, 2011: estimates based on 177 cancer registries, Thorac cancer 7 (2) (2016) 232-237.
[2] W. Chen, R. Zheng, P.D. Baade, S. Zhang, H. Zeng, F. Bray, A. Jemal, X.Q. Yu, J. He, Cancer statistics in China, 2015, CA A Cancer J. Clin. 66 (2) (2016) 115-132.
[3] S. Ohashi, S. i. Miyamoto, O. Kikuchi, T. Goto, Y. Amanuma, M. Muto, Recent advances from basic and clinical studies of esophageal squamous cell carcinoma, Gastroenterology 149 (7) (2015) 1700-1715.
[4] A. Pennathur, M.K. Gibson, B.A. Jobe, J.D. Luketich, Oesophageal carcinoma, Lancet 381 (9864) (2013) 400e412 (London, England).
[5] C.P. Ponting, P.L. Oliver, W. Reik, Evolution and functions of long noncoding RNAs, Cell 136 (4) (2009) 629-641.
[6] E.A. Gibb, C.J. Brown, W.L. Lam, The functional role of long non-coding RNA in human carcinomas, Mol. Canc. 10 (1) (2011) 38.
[7] L.L. Chen, G.G. Carmichael, Long noncoding RNAs in mammalian cells: what, where, and why? RNA 1 (1) (2010) 2-21.
[8] A.M. Schmitt, H.Y. Chang, Long noncoding RNAs in cancer pathways, Cancer Cell 29 (4) (2016) 452-463.
[9] W. Wang, Y. Zhu, S. Li, X. Chen, G. Jiang, Z. Shen, Y. Qiao, L. Wang, P. Zheng, Y. Zhang, Long noncoding RNA MALAT1 promotes malignant development of esophageal squamous cell carcinoma by targeting b-catenin via Ezh2, Oncotarget 7 (18) (2016) 25668-25682.
[10] X. Chen, H. Han, Y. Li, Q. Zhang, K. Mo, S. Chen, Upregulation of long non-coding RNA HOTTIP promotes metastasis of esophageal squamous cell carcinoma via induction of EMT, Oncotarget 7 (51) (2016) 84480-84485.
[11] E. Zhang, L. Han, D. Yin, X. He, L. Hong, X. Si, M. Qiu, T. Xu, W. De, L. Xu, Y. Shu, J. Chen, H3K27 acetylation activated-long non-coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma, Nucleic Acids Res. 45 (6) (2016) 3086-3101.
[12] X. Liu, D. Li, W. Zhang, M. Guo, Q. Zhan, Long non-coding RNA gadd7 interacts with TDP-43 and regulates Cdk6 mRNA decay, EMBO J. 31 (23) (2012) 4415-4427.
[13] Y. Zhao, Y. Liu, L. Lin, Q. Huang, W. He, S. Zhang, S. Dong, Z. Wen, J. Rao, W. Liao, M. Shi, The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1, Mol. Canc. 17 (2018) 69.
[14] Y. Lan, X. Xiao, Z. He, Y. Luo, C. Wu, L. Li, X. Song, Long noncoding RNA OCC-1 suppresses cell growth through destabilizing HuR protein in colorectal cancer, Nucleic Acids Res. 46 (11) (2018) 5809-5821.
[15] Q. Zou, E. Zhou, F. Xu, D. Zhang, W. Yi, J. Yao, A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration, J. Cell. Biochem. 119 (2) (2017) 2189-2199.
[16] Y. Song, C. Liu, X. Liu, J. Trottier, M. Beaudoin, L. Zhang, C. Pope, G. Peng, O. Barbier, X. Zhong, L. Li, L. Wang, H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of EpCAM, Hepatology (Baltimore, Md.) 66 (4) (2017) 1183-1196.
[17] E.A. Lendermon, T.A. Coon, J.S. Bednash, N.M. Weathington, J.F. McDyer, R.K. Mallampalli, Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation, Respir. Res. 18 (2017) 131.
[18] W. Zang, T. Wang, Y. Wang, X. Chen, Y. Du, Q. Sun, M. Li, Z. Dong, G. Zhao, Knockdown of long non-coding RNA TP73-AS1 inhibits cell proliferation and induces apoptosis in esophageal squamous cell carcinoma, Oncotarget 7 (15) (2016) 19960-19974.
[19] Y.S. Tong, X.W. Wang, X.L. Zhou, Z.H. Liu, T.X. Yang, W.H. Shi, H.W. Xie, J. Lv, Q.Q. Wu, X.F. Cao, Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma, Mol. Canc. 14 (1) (2015) 3.
[20] W. Wang, X. He, Z. Zheng, X. Ma, X. Hu, D. Wu, M. Wang, Serum HOTAIR as a novel diagnostic biomarker for esophageal squamous cell carcinoma, Mol. Canc. 16 (2017) 75.
[21] Y. Wu, L. Hu, Y. Liang, J. Li, K. Wang, X. Chen, H. Meng, X. Guan, K. Yang, Y. Bai, Up-regulation of lncRNA CASC9 promotes esophageal squamous cell carcinoma growth by negatively regulating PDCD4 expression through EZH2, Mol. Canc. 16 (2017) 150.
[22] C. Lin, N. Zhang, Y. Wang, Y. Wang, C. Guo, E. Nice, E. Zhang, L. Yu, M. Zhang, C. Liu, L. Hu, J. Hao, W. Qi, H. Xu, Functional role of A novel long noncoding RNA TTN-AS1 in esophageal squamous cell carcinoma progression and metastasis, Clin. Cancer Res. 24 (2) (2017) 486-498.
[23] X.D. Zhang, G.W. Huang, Y.H. Xie, J.Z. He, J.C. Guo, X.E. Xu, L.D. Liao, Y.M. Xie, Y.M. Song, E.M. Li, L.Y. Xu, The interaction of lncRNA EZR-AS1 with SMYD3 maintains overexpression of EZR in ESCC cells, Nucleic Acids Res. 46 (4) (2017) 1793-1809.
[24] J.G. van Kampen, O. van Hooij, C.F. Jansen, F.P. Smit, P.I. van Noort, I.J. Schultz, R.Q. Schaapveld, J.A. Schalken, G.W. Verhaegh, MicroRNA-520f reverses epithelial-to-mesenchymal transition by targeting ADAM9 and TGFBR2, Cancer Res. 77 (8) (2017) 2008-2017.
[25] L. Adam, M. Zhong, W. Choi, W. Qi, M. Nicoloso, A. Arora, G. Calin, H. Wang, A. Siefker-Radtke, D. McConkey, M. Bar-Eli, C. Dinney, miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy, Clin. Cancer Res. 15 (16) (2009) 5060-5072.
[26] J.H. Taube, J.I. Herschkowitz, K. Komurov, A.Y. Zhou, S. Gupta, J. Yang, K. Hartwell, T.T. Onder, P.B. Gupta, K.W. Evans, B.G. Hollier, P.T. Ram, E.S. Lander, J.M. Rosen, R.A. Weinberg, S.A. Mani, Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes, Proc. Natl. Acad. Sci. U.S.A. 107 (35) (2010) 15449-15454.
[27] A.E. Sayan, T.R. Griffiths, R. Pal, G.J. Browne, A. Ruddick, T. Yagci, R. Edwards, N.J. Mayer, H. Qazi, S. Goyal, S. Fernandez, K. Straatman, G.D.D. Jones, K.J. Bowman, A. Colquhoun, J.K. Mellon, M. Kriajevska, E. Tulchinsky, SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer, Proc. Natl. Acad. Sci. U.S.A. 106 (35) (2009) 14884-14889.
[28] H. Hirata, Y. Hinoda, V. Shahryari, G. Deng, K. Nakajima, Z.L. Tabatabai, N. Ishii, R. Dahiya, Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with mir-205, Cancer Res. 75 (7) (2015) 1322-1331.
[29] C. Cao, J. Sun, D. Zhang, X. Guo, L. Xie, X. Li, D. Wu, L. Liu, The long intergenic noncoding RNA UFC1, a target of MicroRNA 34a, interacts with the mRNA stabilizing protein HuR to increase levels of b-catenin in HCC cells, Gastroenterology 148 (2) (2015) 415-426, e418.
[30] Y. Qi, H.S. Ooi, J. Wu, J. Chen, X. Zhang, S. Tan, Q. Yu, Y.Y. Li, Y. Kang, H. Li, Z. Xiong, T. Zhu, B. Liu, Z. Shao, X. Zhao, MALAT1 long ncRNA promotes gastric cancer metastasis by suppressing PCDH10, Oncotarget 7 (2016) 12693-12703.
[31] E.J. Tan, K. Kahata, O. Idås, S. Thuault, C.H. Heldin, A. Moustakas, The high mobility group A2 protein epigenetically silences the Cdh1 gene during epithelial-to-mesenchymal transition, Nucleic Acids Res. 43 (1) (2015) 162-178.
[32] J.H. Chen, L.Y. Zhou, S. Xu, Y.L. Zheng, Y.F. Wan, C.P. Hu, Overexpression of lncRNA HOXA11-AS promotes cell epithelial-mesenchymal transition by repressing miR-200b in non-small cell lung cancer, Cancer Cell Int. 17 (2017) 64.
