Carbon Ion Radiotherapy Combined with Immunotherapy for Recurrent Nasopharyngeal Carcinoma in Specia
Carbon Ion Radiotherapy Combined with Immunotherapy for Recurrent Nasopharyngeal Carcinoma in Special Site: A Case Report
LI Xiao-jun, HU Ting-chao, ZHANG Yan-shan, YE Yan-cheng, ZHOU Jun-nian, MA Shu-ping, ZHANG Tian-e, WANG Yan, WANG Jian
Keywords: Carbon ion radiotherapy; Pembrolizumab; Recurrent nasopharyngeal carcinoma; Efficacy
Chinese Library Classification Number: R739.63
Document Code: B
Article ID: 1671-170X(2022)12-1046-06
doi: 10.11735/j.issn.1671-170X.2022.12.B013
Nasopharyngeal carcinoma (NPC) exhibits a distinct geographical epidemiological pattern, with southern China and Southeast Asia being high-incidence regions globally. According to statistics, the incidence of NPC in southern China is as high as 30.29 per 100,000 in males and 13.09 per 100,000 in females[1]. Radiotherapy is the primary treatment modality for NPC. With advancements in imaging diagnostics, intensity-modulated radiotherapy (IMRT), chemotherapy, and targeted therapy, survival rates for early and locally advanced NPC have significantly improved, with a favorable prognosis and a 5-year survival rate of 85%–90%[2-4]. However, approximately 10% of patients still experience residual or recurrent disease at the primary and/or regional sites. The Expert Consensus on Diagnosis of Recurrent or Metastatic Nasopharyngeal Carcinoma (2018) indicates[5] that the main treatment modalities for recurrent NPC include re-irradiation, salvage surgery, chemotherapy, targeted therapy, and immunotherapy. Re-irradiation techniques primarily include IMRT, stereotactic radiotherapy (SRT), as well as proton and carbon ion radiotherapy[6-7]. However, NPC tumor tissues often exhibit insufficient blood supply and contain hypoxic cells that are resistant to chemoradiotherapy. Some cancer cells can evade radiation-induced damage and maintain viability through anaerobic glycolysis[8]. Tumors invading areas such as the skull base, clivus, and cavernous sinus may not receive radical radiation doses due to normal tissue dose constraints, becoming a source of recurrence. Carbon ion radiotherapy deposits energy at the end of its range, forming a Bragg peak with minimal lateral scattering and sharp dose fall-off, allowing precise treatment (on a millimeter scale) and minimizing radiation damage to surrounding tissues. Carbon ion beams also possess a high relative biological effectiveness (RBE), causing double-strand DNA breaks that are difficult to repair. These characteristics make carbon ions advantageous for treating radiation-insensitive, anatomically complex recurrent head and neck tumors. Multiple studies have confirmed that immune checkpoint inhibitors (ICIs) combined with standard chemotherapy regimens for recurrent and metastatic NPC can produce synergistic effects, with efficacy superior to chemotherapy alone, potentially related to chemotherapy's impact on tumor neoantigen exposure and the tumor immune microenvironment[9]. Therefore, in recent years, researchers have been exploring more effective treatment strategies. Our center employed a combination of carbon ion radiotherapy and the immune checkpoint inhibitor pembrolizumab (Keytruda) on a 6-week schedule to treat a case of NPC with skull base recurrence involving the atlas and tentorium cerebelli after prior radiotherapy. Despite the challenging recurrence location, complete tumor disappearance was achieved within 6 months post-treatment, and the patient remained disease-free for 16 months. This case is reported below.
1 Clinical Data
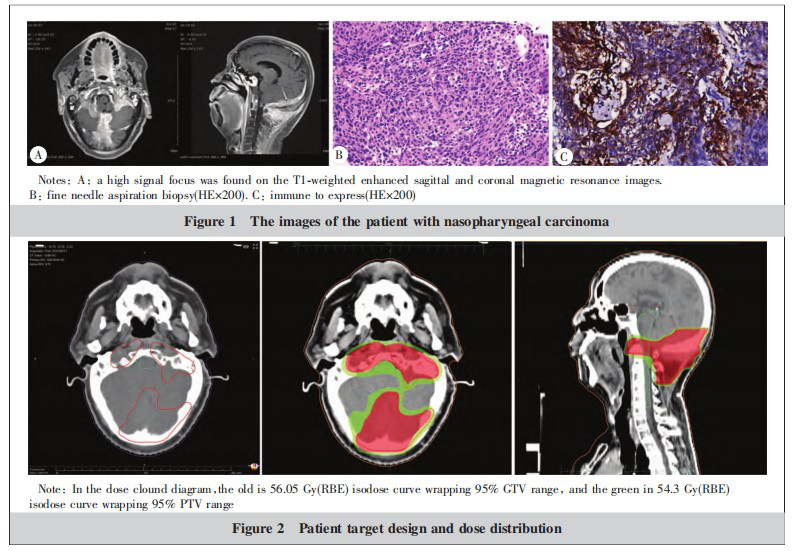
A 55-year-old male patient was admitted with the chief complaint of "skull base recurrence confirmed 1 year after 4 years post-NPC radiotherapy." In December 2017, symptoms of "nasal obstruction and turbid nasal discharge" led to nasopharyngeal endoscopy, which revealed raised neoplastic lesions with an irregular surface bilaterally in the nasopharynx. Pathological examination indicated: (nasopharynx) poorly differentiated squamous cell carcinoma (non-keratinizing, undifferentiated type). Immunohistochemistry (IHC): CK(+), CK5/6(++), P40(+), LCA (lymphocytes+), P53 (15%+), Ki-67 (50%+), PD-1(-), PD-L1(-). EBER in situ hybridization was positive. Clinical staging was cT4N1M0, stage IVA. From December 2017 to January 2018, the patient received 3 cycles of neoadjuvant chemotherapy with docetaxel + nedaplatin (TP regimen). From January to February 2018, definitive volumetric modulated arc therapy (VMAT) was administered for NPC, with a prescribed dose of DT 70 Gy/31 F to the gross tumor volume (GTV). Concurrent weekly nimotuzumab targeted therapy was given for 6 weeks during radiotherapy. Post-radiotherapy evaluation showed a complete response (CR). From March to July 2018, the patient received 6 cycles of oral tegafur-gimeracil-oteracil potassium (S-1) chemotherapy. From November 2018 to August 2020, intermittent oral capecitabine was administered as "metronomic chemotherapy." In September 2020, nasopharyngeal MRI showed mild thickening of the nasopharyngeal wall, unchanged from previous scans. Skull base bone destruction suggested tumor infiltration. Occipital bone destruction with a surrounding soft tissue mass measuring approximately 66 mm × 64 mm × 51 mm indicated recurrent metastatic disease with invasion. From September to November 2020, 3 cycles of cisplatin + fluorouracil (DF regimen) combined with cetuximab chemotherapy were administered. From November to December 2020, 2 cycles of cisplatin + albumin-bound paclitaxel (TP regimen) chemotherapy were given. From December 2020 to July 2021, 9 cycles of cisplatin + gemcitabine (GP regimen) combined with nivolumab and cetuximab were administered, but efficacy evaluation indicated progressive disease (PD). In September 2021, nasopharyngeal MRI (Figure 1A) showed recurrence in the clivus post-NPC radiotherapy, with occipital bone destruction and intra- and extracranial soft tissue mass formation, partially enveloping the atlas, indicating recurrent metastasis. Pathological examination of a puncture biopsy from the occipital lesion indicated: fibrous tissue with metastatic carcinoma. Combined with IHC, in situ hybridization results, and clinical history, nasopharyngeal carcinoma metastasis was considered. IHC results (Figure 1B) showed: CK5/6(+), Ki-67 (approximately 20%+), LCA(-), P40(+). In situ hybridization showed: EBER(+). Immune expression (Figure 1C) showed: PD-L1 tumor proportion score (TPS) 50%–60%, combined positive score (CPS) 60. The clinical diagnosis was: recurrent undifferentiated non-keratinizing nasopharyngeal carcinoma after chemoradiotherapy, post-chemotherapy and immunotherapy, rcT4N0M1 stage IVB, ECOG score 1.
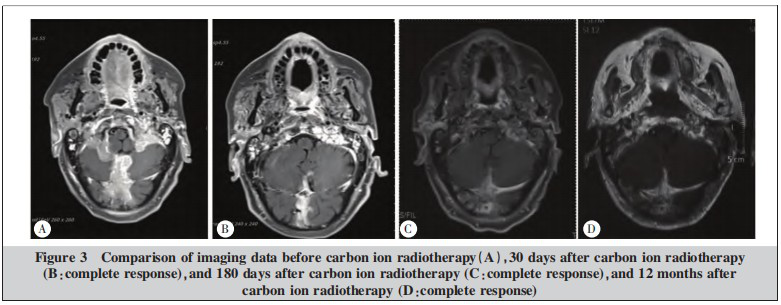
After comprehensive examination and evaluation, the patient received active beam delivery carbon ion radiotherapy in September 2021. The treatment plan was developed using the ciPlan1.0 system (Institute of Modern Physics, Lanzhou, China). Target delineation (Figure 2A): GTV was delineated as the visible recurrent and invasive lesions on CT and MRI. PTV was created by expanding the CTV by 3 mm. Organs at risk (OARs) delineated included the spinal cord, brainstem, cerebellum, temporomandibular joints, and mandible. A 3D spot-scanning technique was used for radiotherapy, employing left and right opposing fields to irradiate the lesion. The total dose was 57 Gy(RBE)/19 F, 3.0 Gy(RBE)/F, 5 F/week (Figure 2). Dose-volume histogram (DVH) analysis indicated that the 56.05 Gy(RBE) isodose line covered 95% of the GTV, and the 54.3 Gy(RBE) isodose line covered 95% of the PTV. The maximum dose (Dmax) to the brainstem was 10.34 Gy(RBE). Comparison of imaging data before carbon ion treatment, and at 30 days, 180 days, and 12 months post-treatment showed that the efficacy reached complete response (CR) at 30 days post-treatment (Figure 3). Within 30 days after carbon ion treatment, mild erythema and dry desquamation occurred in the skin of the occipital irradiation field, graded as grade 1 radiation skin injury according to RTOG acute radiation injury criteria. No adverse reactions greater than grade 2 were observed. During carbon ion treatment, an immunoradiotherapy combination model was adopted. Based on the KEYNOTE-028 study, third-line single-agent pembrolizumab salvage therapy was used, adopting the latest dosing regimen: pembrolizumab was switched from 200 mg every 3 weeks to 400 mg every 6 weeks, and this regimen continues to date. As of September 2022 (12 months after tumor disappearance), the patient remains alive and disease-free. The next phase involves continuing with a 5-year long-term follow-up plan.
2 Discussion
Treatment methods for locally or regionally recurrent NPC include surgery, re-irradiation, chemotherapy, targeted therapy, and immunotherapy, each with limitations as a single modality. Re-irradiation is an effective salvage treatment, particularly for patients with a recurrence interval exceeding one year. Re-irradiation requires thorough assessment of the initial radiotherapy dose, time to recurrence, normal tissue tolerance, and potential acute and late toxicities from the second course of radiation. Stage T1 and T2 recurrent NPC can be treated with re-irradiation or minimally invasive surgery. Due to the localized nature of the lesions, re-irradiation yields good results with low associated toxicity. Minimally invasive surgery, especially endoscopic nasopharyngectomy and Da Vinci robot-assisted minimally invasive nasopharyngectomy, plays a significant role in treating early recurrent NPC. The comparative efficacy of these two approaches requires further investigation. Re-irradiation for stage T3 and T4 recurrent NPC patients is associated with high toxicity, poor response, and very high treatment-related mortality, with over 40% of deaths attributed to mucosal necrosis or massive hemorrhage. Difficulty eating and radiation encephalopathy are also common causes of grade 5 toxicities. Proton and heavy ion therapy show promise as ideal treatment modalities. Concurrent chemotherapy may improve the prognosis of patients with recurrent NPC but can exacerbate radiotherapy toxicity. Whether to use induction chemotherapy warrants further investigation through prospective randomized controlled trials. Immune checkpoint inhibitors have shown preliminary efficacy in treating recurrent metastatic NPC, but the response rate of PD-1/PD-L1 inhibitors alone is relatively low. Combination with chemotherapy is expected to significantly improve outcomes for recurrent NPC patients, though long-term definitive efficacy needs further confirmation through research. Radiotherapy combined with immunotherapy has demonstrated synergistic effects in treatment-naive tumors, but reports on radiotherapy combined with immunotherapy for recurrent NPC, particularly immunotherapy combined with carbon ions, are scarce in clinical practice.
Re-irradiation for locally recurrent NPC is challenging because previous radiation doses have often reached the tolerance limits of surrounding normal tissues, necessitating a balance between local salvage therapy and minimizing treatment toxicity. Li Jiaxin et al.[10] collected data from 337 patients with first recurrence of NPC. Statistical analysis of imaging showed higher recurrence rates in areas such as the skull base, paranasal sinuses, cranial nerves, cavernous sinus, intracranial space, pterygopalatine fossa, infratemporal fossa, and orbital apex. These regions, due to their functional importance and complex anatomy, are subject to normal tissue dose constraints, leading to low-dose areas where tumor cells often sustain sublethal damage, repair themselves, survive, and continue proliferating, causing recurrence. Re-irradiation after recurrence is often limited by dose constraints for organs at risk during the second course. Previous reports exist on the efficacy and toxicity of carbon ion therapy for recurrent NPC. In 2016, the Shanghai Proton and Heavy Ion Center reported the protocol for a Phase I/II clinical trial evaluating carbon ion radiotherapy combined with chemotherapy as salvage treatment for locally recurrent NPC[11], expecting improved efficacy and reduced toxicity compared to photon radiotherapy, aiming to increase the 2-year overall survival rate from a historical 50% to at least 70%. In 2018, Hu et al.[12] published initial results: from May 2015 to August 2017, 75 patients with recurrent NPC received salvage treatment with intensity-modulated carbon ion radiotherapy (IMCR). Stages I, II, III, and IVA/B were 4, 14, 29, and 28 cases, respectively. The IMCR dose was 50–66 Gy(RBE). The 1-year overall survival rate was 98.1%, the local-regional recurrence-free survival rate was 86.6%, and the distant metastasis-free survival rate was 96.2%. No acute toxicity reactions above grade 2 occurred during treatment. Late severe toxicities (grade 3 or 4) were uncommon, including mucosal necrosis (7 cases), dry mouth (1 case), and temporal lobe necrosis (1 case). Currently, our center has also accumulated a certain number of clinical cases of carbon ion therapy for recurrent head and neck tumors, with encouraging treatment outcomes.
Analysis of marginal dose fall-off in heavy ion radiotherapy by Ma Xiaoyun et al. from our center[13] showed that due to the unique physical properties of heavy ion beams, the dose fall-off at the target edge is very rapid. At 5 mm beyond the 95% isodose line at the posterior edge of the heavy ion beam, the dose attenuates by 70%–80%. At a distance of 1 mm beyond the field edge, the dose attenuates by approximately 8%–19%[14]. Compared to X/γ-ray radiotherapy, heavy ion beams exhibit a faster dose fall-off. Heavy ions can enable safe and adequate radiotherapy for targets very close to organs at risk. In the case we reported, the recurrence was located at the skull base involving the atlas and tentorium cerebelli. The target volume was irregularly shaped, adjacent to the cerebellum and circumferentially surrounding the medulla oblongata, making dose distribution unachievable with IMRT or SRT. Carbon ions offer physical and biological advantages. Using carbon ion spot-scanning radiotherapy, re-irradiation was perfectly achieved. Firstly, the sharp Bragg peak of the monoenergetic beam was spread out using a mini-ridge filter (miniSOBP)[15]. During active beam delivery, the particle energy was altered to change the penetration depth of the ion beam, and a magnetic scanning system was used to guide the pencil beam for 3D scanning and conformal therapy of the tumor target[16]. The high-dose region (Bragg peak) was adjusted to conform to the tumor target. At 3 mm beyond the 95% isodose line at the field edge, the dose rapidly fell by 50%–60%. Furthermore, carbon ions have a high RBE in the Bragg peak region, with the greatest difference in RBE values between the peak and plateau regions, sparing the medulla oblongata and cerebellum located in the plateau region from excessive damage.
Currently, most recurrent NPC cases are unsuitable for conventional local re-treatment or surgery. The mainstream treatment strategy is palliative systemic chemotherapy. For patients who fail first-line platinum-based regimens, monotherapy with agents not used in the first line is typically chosen. In 2020, four domestic immune monotherapy studies—POLARIS-02 (toripalimab), CAPTAIN (camrelizumab), BGB-A317-102 (tislelizumab), and AK-105 (penpulimab)—reported data on recurrent and metastatic NPC after standard treatment failure. The KEYNOTE-028 study[17] reported the anti-tumor activity and safety evaluation of pembrolizumab in recurrent metastatic NPC (PD-L1 TPS ≥1%). With a median follow-up of 20 months, the objective response rate was 25.9%. In the case we reported, after recurrence in September 2020, DF, TP, and GP regimen chemotherapies were sequentially used, combined with the targeted drug cetuximab and the immune checkpoint inhibitor nivolumab, yet the tumor remained uncontrolled. Upon admission, a biopsy of the occipital recurrent lesion retested immune expression, showing PD-L1 TPS of 50%–60% and CPS of 60. For this patient who failed first and second-line therapies, third-line single-agent pembrolizumab salvage therapy was adopted based on the KEYNOTE-028 study, alongside an evaluation of the feasibility of carbon ion radiotherapy, leading to combination radiotherapy. A dose conversion was implemented during drug use: pembrolizumab was switched from 200 mg every 3 weeks to 400 mg every 6 weeks. This did not induce or exacerbate immune-related adverse events, consistent with the results of a dose conversion study in advanced non-small cell lung cancer published by Japanese researchers in the Journal of Thoracic Oncology in July 2022[18].
Basic research indicates that the combination of immunotherapy and radiotherapy provides synergistic benefits through two main aspects: (1) Radiotherapy can activate anti-tumor immunity and remodel the immune microenvironment, enhancing the anti-tumor activity of immunotherapy[19-21]. Radiotherapy not only induces adaptive anti-tumor immune responses but also modifies the properties of key immune cells in the microenvironment, transforming poorly immunogenic tumors into more immunogenic ones, thereby enhancing anti-tumor immune reactions. Both low and high-dose radiotherapy promote the upregulation of immunostimulatory and immunosuppressive immune cells. (2) Immunotherapy can sensitize tumors to radiotherapy, making tumor cells more susceptible to radiation[22]. Abnormal and dysfunctional tumor vasculature leads to tumor hypoxia and subsequent radioresistance. Immune checkpoint inhibitors induce vascular normalization through T-cell-dependent pathways, reverse local tumor hypoxia and cell infiltration, and through this improvement, increase the radiosensitivity of tumor tissue, further promoting tumor regression. This effect is commonly observed in hypofractionated SRT[23] and with high linear energy transfer (LET) radiation, such as carbon ion radiotherapy[24]. Because carbon ion radiotherapy directly causes double-strand DNA breaks in tumor cells, it more readily induces systemic anti-tumor immunity, enhances tumor-associated antigen presentation, and improves immune system recognition of tumors. Thus, combining carbon ions with anti-PD-1/PD-L1 monoclonal antibodies can produce synergistic effects and induce abscopal effects. Previously, our center reported a case of abscopal effect after carbon ion radiation therapy alone for malignant thymoma[25], where significant shrinkage was observed both inside and outside the treatment area at the end of therapy.
Compared to re-irradiation with IMRT, both proton and heavy ion radiotherapy can further reduce damage to normal tissues[26-27]. Although randomized controlled trials are currently lacking, small-sample retrospective studies suggest that proton and heavy ion radiotherapy techniques hold significant application promise for recurrent and metastatic NPC[28]. For this case of recurrent NPC treated with carbon ions combined with immune checkpoint inhibitors, excellent results were achieved without adverse reactions greater than grade 2. Complete tumor disappearance was achieved post-treatment, and the patient remained disease-free for 12 months, with a current overall survival of 13 months, successfully accomplishing re-irradiation for recurrence in a special site. This demonstrates the superior physical and biological advantages of carbon ions. For challenging cases where organs at risk are extremely close to the target, carbon ion radiotherapy can meet the target dose requirements while reducing the dose to adjacent critical organs below tolerance limits.
In conclusion, carbon ion therapy combined with immunotherapy may become a safe and effective treatment method for recurrent and metastatic nasopharyngeal carcinoma. However, longer follow-up time and more data from clinical observation are needed to evaluate its efficacy and late toxicities.
References
[1] Zhang LF, Li YH, Xie SH, et al. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period cohort analysis[J]. Chin J Cancer, 2015, 34(8): 350–357.
[2] Becker-Schiebe M, Christiansen H. Update zur kombinierten radio-, radiochemo- und alleinigen Chemotherapie bei der multimodalen Therapie des Nasopharynxkarzinoms--eine MAC-NPC-Metaanalyse[Update on combined radio-, radiochemo-, and chemotherapy alone in multimodal therapy of nasopharyngeal carcinoma --a MAC NPC meta-analysis][J]. Strahlenther Onkol, 2015, 191(12): 991–993.
[3] Yang L, Hong S, Wang Y, et al. Development and external validation of nomograms for predicting survival in nasopharyngeal carcinoma patients after definitive radiotherapy[J]. Sci Rep, 2015, 26(5): 15638.
[4] Perri F, Della Vittoria Scarpati G, et al. Combined chemoradiotherapy in locally advanced nasopharyngeal carcinomas[J]. World J Clin Oncol, 2013, 4(2): 47–51.
[5] 李金高,陈晓钟,林少俊,等. 鼻咽癌复发、转移诊断专家共识[J]. 中华放射肿瘤学杂志,2018,27(1): 7–15.
[6] Helbig L, Koi L, Brüchner K, et al. Hypoxia-inducible factor pathway inhibition resolves tumor hypoxia and improves local tumor control after single-dose irradiation[J]. Int J Radiat Oncol Biol Phys, 2014, 88(1): 159–166.
[7] Lee AWM, Ng WT, Chan JYW, et al. Management of locally recurrent nasopharyngeal carcinoma[J]. Cancer Treat Rev, 2019, 79(9): 101890.
[8] Dizman A, Coskun-Breuneval M, Altinisik-Inan G, et al. Reirradiation with robotic stereotactic body radiotherapy for recurrent nasopharyngeal carcinoma[J]. Asian Pac J Cancer Prev, 2014, 15(8): 3561–3566.
[9] Meric-Bernstam F, Larkin J, et al. Enhancing anti-tumour efficacy with immunotherapy combinations[J]. Lancet, 2021, 397(10278): 1010–1022.
[10] 李嘉欣,卢泰祥,黄莹,等. 337例复发鼻咽癌患者的临床特征[J]. 癌症,2010,29(1): 82–86.
[11] Kong L, Gao J, Hu J, et al. Phase I/II trial evaluating concurrent carbon-ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma[J]. Chin J Cancer, 2016, 35(1): 101.
[12] Hu J, Bao C, Gao J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: Initial results[J]. Cancer, 2018, 124(11): 2427–2437.
[13] 马霄云,张雁山,张梦灵,等. 重离子放射治疗靶区边缘剂量跌落分析[J]. 肿瘤学杂志,2021,27(5): 390–394.
[14] 王金媛,鞠忠建,王小深,等. 椎体转移瘤立体定向放射治疗剂量跌落梯度分析[J]. 中国医学装备,2015,12(9): 1–5.
[15] 盛尹祥子,Kambiz Shanazi,王巍伟,等. 点扫描质子束治疗机头的蒙特卡罗模拟和验证[J]. 中华放射医学与防护杂志,2019(8): 635–640.
[16] 李小波,江柳清,吴晓东,等. 高剂量格栅状放疗技术及应用[J]. 中华放射肿瘤学杂志,2018,27(6): 624–628.
[17] Hsu C, Lee SH, Ejadi S, et al. Safety and Antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study[J]. J Clin Oncol, 2017, 35(36): 4050–4056.
[18] Higashiyama RI, Yoshida T, Yagishita S, et al. Safety implications of switching Pembrolizumab dosage from 200 mg every 3 weeks to 400 mg every 6 weeks in patients with advanced NSCLC[J]. J Thorac Oncol, 2022, 17(10): 1227–1232.
[19] Keam S, Gill S, Ebert MA, et al. Enhancing the efficacy of immunotherapy using radiotherapy[J]. Clin Transl Immunology, 2020, 9(9): e1169.
[20] Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach[J]. Nat Rev Clin Oncol, 2016, 13(8): 516–524.
[21] Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice[J]. CA Cancer J Clin, 2017, 67(1): 65–85.
[22] Wang Y, Liu ZG, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy[J]. Clin Cancer Res, 2019, 25(6): 1709–1717.
[23] Xing D, Siva S, Hanna GG. The abscopal effect of stereotactic radiotherapy and immunotherapy: fool's gold or El Dorado[J]. Clin Oncol (R Coll Radiol), 2019, 31(7): 432–443.
[24] Harada K, Nonaka T, Hamada N, et al. Heavy-ion-induced bystander killing of human lung cancer cells: role of gap junctional intercellular communication[J]. Cancer Sci, 2009, 100(4): 684–688.
[25] Zhang YS, Zhang YH, Li XJ, et al. Bystander effect and abscopal effect in recurrent thymic carcinoma treated with carbon-ion radiation therapy: a case report[J]. World J Clin Cases, 2021, 9(22): 6538–6543.
[26] Akbaba S, Ahmed D, Lang K, et al. Results of a combination treatment with intensity modulated radiotherapy and active raster-scanning carbon ion boost for adenoid cystic carcinoma of the minor salivary glands of the nasopharynx[J]. Oral Oncol, 2019, 91(4): 39–46.
[27] Amirul Islam M, Yanagi T, Mizoe JE, et al. Comparative study of dose distribution between carbon ion radiotherapy and photon radiotherapy for head and neck tumor[J]. Radiat Med, 2008, 26(7): 415–421.
[28] Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma[J]. Lancet, 2019, 394(10192): 64–80.
Preliminary Review: Ma Shuqian
Final Review: Liu Wenyu
