A clinical case report on the abscopal effect of carbon ion radiotherapy
A clinical case report on the abscopal effect of carbon ion radiotherapy
Qin Tianyan , Zhang Yihe , Zhang Yanshan , Ye Yancheng, Pan Xin , Wang Xin , Yang Yuling, Ma Tong, Lyu Caixia , Li Pengqing
Department of Radiotherapy, Wuwei Cancer Hospital of Gansu Province , Wuwei 733000,
China Corresponding author: Zhang Yihe ,Email :sipen@ 163. com
Fund program : Scientific and Technological Projects in Gansu Province (23JRRH006)
Radiotherapy is one of the principal modalities for local cancer treatment. Relevant studies have revealed that radiation can induce a specific immunostimulatory effect known as the abscopal effect (AE), which refers to the shrinkage of lesions outside the irradiated field when local tumor sites are targeted by radiation. Carbon ion radiotherapy (CIRT) is currently considered an optimal approach in clinical radiotherapy, offering several advantages over conventional radiotherapy and demonstrating potential to activate stronger immunogenicity in tumor cells [1]. From a physical perspective, carbon ions exhibit a characteristic energy distribution in depth known as the "Bragg peak," resulting in high energy deposition in tissues near the target area, while proximal tissues receive minimal energy. Biologically, carbon ions possess a high linear energy transfer (LET) and exhibit a higher relative biological effectiveness (RBE) compared to photons. The biological effect in the peak region is amplified by at least 2–4 times relative to photon radiotherapy [2–4]. Due to these physical and biological advantages, CIRT is hypothesized to induce a stronger immune response than conventional radiotherapy [5]. Related studies also suggest that the mechanism underlying the abscopal effect involves a series of changes in the immune system triggered by localized radiotherapy [6]. Currently, clinical and mechanistic research on the abscopal effect induced by carbon ion radiotherapy remains limited. Most experimental results indicate that CIRT can elicit a potent pro-immunogenic response and induce more pronounced abscopal effects [5]. This study summarizes and reports three typical cases of the abscopal effect observed since the clinical implementation of the Wuwei Carbon Ion Therapy System, aiming to provide reference for further clinical research on the abscopal effect in carbon ion radiotherapy.
1. Case Presentation
Case 1: A 48-year-old female patient presented with the chief complaint of "palpitations and shortness of breath for one year, 10 years after surgery for malignant thymoma." On February 16, 2009, the patient underwent "extensive resection of thymic tumor + partial resection of the left upper lung lobe, phrenic nerve, and pericardium." Postoperative pathology revealed: (anterosuperior mediastinum) non-keratinizing squamous cell carcinoma of the thymus. Thirty-five days after surgery, the patient received postoperative intensity-modulated radiotherapy with a total dose of 50 Gy in 25 fractions, targeting the original tumor bed and the superior mediastinum. Thereafter, the patient received no further treatment and underwent regular follow-up chest CT scans. In December 2019, the patient again experienced symptoms of palpitations and shortness of breath. A repeat chest CT showed multiple nodules in the left pleura, diaphragmatic crus, and peritoneum, with the largest lesion (6.3 cm × 5.2 cm) located in the left pleura adjacent to the pericardium, along with multiple small lymph nodes in the left cervical root, mediastinum, and retroperitoneum, suggesting recurrent metastasis of thymic carcinoma. The clinical diagnosis was recurrent metastatic thymic carcinoma, classified as T3N0M1a Stage IVA according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. After multidisciplinary team (MDT) consultation, considering the patient’s long disease-free survival following previous surgery and radiotherapy and the indolent biological behavior of the tumor, palliative carbon ion radiotherapy (CIRT) was administered solely to the largest tumor adjacent to the pericardium on the left pleura due to its compressive invasion of the heart, aiming to alleviate the symptoms of palpitations and shortness of breath. The treatment plan involved palliative carbon ion therapy targeting the lesion at the left cardiac margin, with a planned target volume (PTV) dose of 60 Gy(RBE) in 12 fractions, ensuring that 90% of the PTV received the prescription dose. During treatment, the patient only developed mild skin pigmentation at the treatment site, diagnosed as Grade 1 radiation dermatitis according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The skin reaction resolved almost completely two months after treatment completion. A chest CT scan performed on the day of treatment completion revealed not only shrinkage of the irradiated lesion (left pericardial region) but also varying degrees of reduction in metastatic lesions in areas that received low-dose or no irradiation (left chest wall and left diaphragmatic crus). These lesions continued to shrink during follow-up over the subsequent year. The maximum diameters of the pericardial lesion were 6.3 cm, 4.6 cm, and 3.9 cm before treatment, at treatment completion, and one year after treatment, respectively. The left chest wall lesion measured 2.8 cm, 1.9 cm, and 0.8 cm, while the left diaphragmatic crus lesion measured 4.1 cm, 3.9 cm, and 3.4 cm at the same time points. According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1:
• Complete Response (CR) is defined as the disappearance of all target lesions and reduction of lymph node short axis to <10 mm, sustained for at least 4 weeks.
• Partial Response (PR) is defined as a ≥30% decrease in the sum of the longest diameters of target lesions, sustained for at least 4 weeks.
• Progressive Disease (PD) is defined as a ≥20% increase in the sum of the longest diameters compared to the smallest sum observed, an absolute increase of ≥5 mm, or the appearance of new lesions.
• Stable Disease (SD) is defined as neither sufficient shrinkage to qualify as PR nor sufficient increase to qualify as PD.
The efficacy in this case was evaluated as PR (Figure 1).
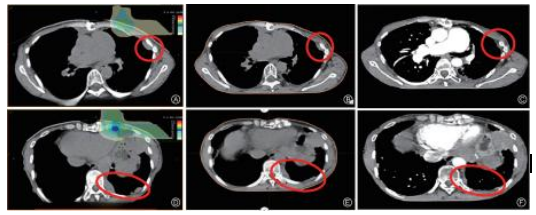
Note: Red circles indicate the lesion sites
Figure 1 Pre-treatment and follow-up imaging data of Case A. Pre-treatment isodose curve coverage and unirradiated chest wall lesions: B. 1 year after treatment of uniradiated chest wall lesions; C. 1.5 years after treatment of uniradiated chest wall lesions; D. Pre-treatment isodose curve coverage and unirradiated diaphragm angle lesions; E. 1 year after treatment of unirradiated diaphragm angle lesions; F. 1.5 years after treatment of unirradiated diaphragm angle lesions
Case 2: An 81-year-old male patient presented with the chief complaint of "multiple lung metastases occurring over 6 months after colon cancer surgery." PET-CT imaging revealed: no significant abnormal metabolism at the anastomotic site following right hemicolectomy; multiple nodules of varying sizes in both lungs with increased metabolism, suggestive of metastases; an irregular high-density nodule beneath the right frontal meninges with lower metabolism than the parenchyma and mild surrounding edema, possibly a meningioma. Chest CT indicated multiple nodular masses in both lungs, some with cavitation, consistent with metastatic disease based on medical history. After informing the patient’s family of the condition, a CT-guided lung biopsy was performed. Postoperative pathology showed (lung biopsy tissue) moderately differentiated adenocarcinoma, considered most consistent with metastatic colon cancer given the medical history (Figure 2). The clinical diagnosis was secondary malignant neoplasm of the lung post-operation for malignant neoplasm of the ascending colon, classified as TN, MW stage according to the AJCC 8th edition staging system. Following multidisciplinary consultation, a treatment plan was formulated and explained to the patient’s family. After obtaining informed consent, the patient underwent carbon ion radiotherapy targeting partial metastatic lesions in the left lung under general anesthesia with high-frequency oscillatory ventilator control. A dose of 50 Gy (RBE) was delivered in a single fraction, and the procedure was completed smoothly. Follow-up examinations at 1, 3, and 9 months post-treatment showed significant shrinkage of the irradiated lesions, with an efficacy evaluation of Partial Response (PR). Other non-irradiated metastatic lesions in the lungs also exhibited varying degrees of reduction (Figure 3).
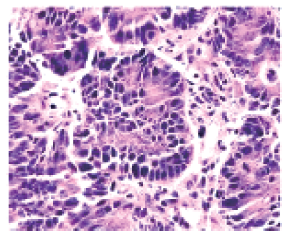
Figure 2 Pathological examination results of
Case 2 before the treatment 10× 100
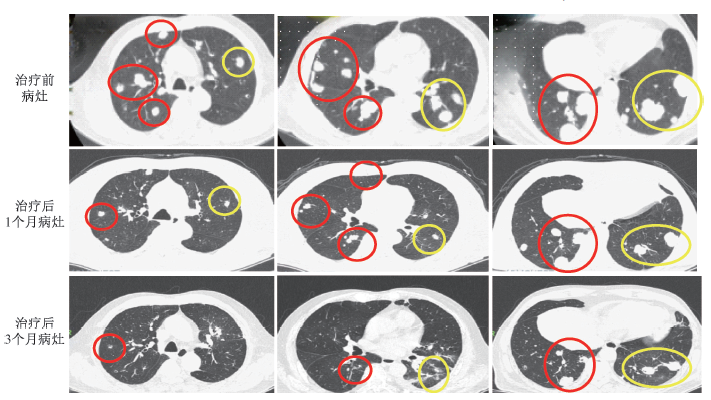
Note: Red circles indicate non-treated lesions; yellow circles indicate treated lesions
Figure 3 Pre⁃teatment and follow⁃up imaging data of Case 2
Case 3: A 53-year-old male patient presented with the chief complaint of "progressive dysphagia for 4 months and hoarseness for 1 month." Chest CT revealed: multiple nodules and masses in the mediastinum, some with fusion, suggestive of metastasis after radical surgery for esophageal cancer. Upper abdominal MRI showed: multiple nodular shadows in the liver with atypical enhancement, some nodules suspicious for halo-like enhancement, not excluding metastasis. Ultrasound-guided biopsy of the retrosternal mass was performed. Pathology indicated: malignant tumor, and immunohistochemical results suggested a mesenchymal-origin malignant tumor. Immunohistochemistry results: CK5/6 (-), CKP (-), Ki67 (index approximately 50%), P40 (-), P63 (-), Vimentin (+), CR (-), HMB-45 (-), S-100 (-), SMA (focal +), Desmin (-), CD117 (-), DOG1 (-) (Figure 4). Clinical diagnosis: Malignant tumor of the mediastinum (mesenchymal origin); secondary malignant tumor of bone; secondary malignant tumor of lymph nodes; secondary malignant tumor of liver; malignant tumor of esophagus (postoperative); squamous cell carcinoma. After multidisciplinary consultation, a treatment plan was formulated and explained to the patient’s family. Following informed consent, the patient received palliative carbon ion therapy for partial mediastinal masses. The first course dose was 30 Gy (RBE) in 10 fractions. Post-treatment CT showed significant shrinkage of the mass and alleviated tracheal compression. The target area was modified, and a second course dose of 18 Gy (RBE) in 6 fractions was administered. After communication with the family, carbon ion therapy was delivered to the left cervical metastatic lymph nodes with a total dose of 52.8 Gy (RBE) in 12 fractions; to the C3 vertebral metastatic lesion with a total dose of 52.8 Gy (RBE) in 12 fractions; to the partial metastatic lesion in liver segment S8 with a total dose of 66 Gy (RBE) in 10 fractions; and stereotactic ablative radiotherapy (SABR) was administered to the right iliac bone and L5 vertebral metastatic lesions with a total dose of 20 Gy in 5 fractions. The treatment proceeded smoothly. Follow-up chest CT and abdominal MRI according to RECIST 1.1 showed efficacy evaluation: Partial Response (PR). Two months after treatment, follow-up imaging revealed that the untreated liver metastatic lesions had disappeared (Figure 5).
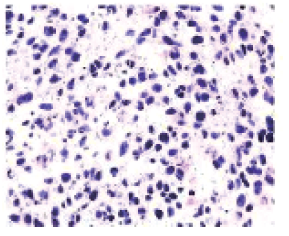
Figure 4 Pathological examination results of Case 3
before the treatment 10× 100

Note: Red circles indicate the lesion sites
Figure 5 Pre⁃treatment and follow⁃up imaging data of Case 3
A. Patient imaging of the carbon ion therapy color washing;
B. Pre⁃treatment of treated lesions ; C. 2⁃month after treatment
of treated lesions ; D. Pre⁃treatment of untreated liver lesions ;
E. 2⁃month after treatment of untreated liver lesions
2. Discussion
In 1953, Mole first proposed the concept of the abscopal effect during abdominal irradiation experiments in rats [5,7]. In 1975, a case of cutaneous reticular cell sarcoma reported regression of non-irradiated metastatic lesions following radiotherapy, and subsequent literature further documented this phenomenon [8-12]. The abscopal effect refers to the shrinkage or even disappearance of tumor lesions outside the irradiated field when localized radiation is delivered to a tumor site [13]. Relevant studies suggest that the underlying mechanism may involve radiation-induced activation of the immune system, release of tumor-associated antigens, and enhanced immunogenicity within the tumor microenvironment. This triggers a systemic anti-tumor immune response, leading to growth inhibition of tumors beyond the irradiated area [13-15]. Alternatively, in addition to directly killing tumor cells, localized radiotherapy may stimulate immune cells to release pro-inflammatory factors, inducing chromosomal breaks or aberrations in non-irradiated tumor cells, thereby mediating immune-driven tumor cell killing and resulting in regression of lesions outside the radiation field [13]. Furthermore, the occurrence and development of the abscopal effect are influenced by factors such as radiation dose, fractionation mode, type of immunotherapy, and tumor characteristics. Notably, tumor immunogenicity is a critical factor in the manifestation of this effect [15-16].
Relevant studies have reported that proton, alpha particle, and carbon ion radiotherapy can more effectively enhance tumor immunogenicity [15]. Carbon ion radiotherapy (CIRT) is considered the most ideal technique in clinical radiotherapy for tumors. Compared with conventional photon radiotherapy, CIRT can induce a stronger anti-tumor immune response [1]. In this study, the thymoma patient showed significant shrinkage of non-irradiated tumors one year after receiving carbon ion therapy. The thymus is an immune organ, and combined with the physical and biological advantages of carbon ions—such as high linear energy transfer (LET) and higher relative biological effectiveness (RBE) compared to photons—CIRT is more capable of inducing a stronger immune response [2-4, 17]. In their study on the biological mechanisms of the abscopal effect (AE) induced by radiotherapy, Demaria et al. [18] also noted that AE does not occur in immunodeficient mice. Other studies have similarly suggested that the mechanism underlying the abscopal effect involves a series of changes in the human immune system triggered by localized radiotherapy [6]. Therefore, it can be inferred that in thymoma patients receiving carbon ion irradiation, the thymus—as an immune organ—acts as a mediator for initiating the abscopal effect, enhancing the presentation of tumor-associated antigens and improving immune recognition of tumors, thereby facilitating the occurrence of the abscopal effect [19]. Sharabi et al. [20] also reported that hypofractionated therapy and high-LET radiation can enhance antigen presentation and T-cell recognition of tumor-associated antigens, making the abscopal effect more likely to occur. In this study, the patient with "multiple lung metastases after colon cancer surgery" received a single high dose of 50 Gy (RBE) carbon ion irradiation to partial metastatic lesions in the left lung. Follow-up imaging showed not only a partial response (PR) in the irradiated lesions but also shrinkage of non-irradiated lung metastases, indicating the occurrence of the abscopal effect. This result is consistent with the findings reported by Sharabi et al. [20]. Among the cases observed in this study, the thymoma patient and the patient with mediastinal malignant tumor (mesenchymal origin) received multiple moderate-dose radiation fractions, while the patient with post-colon cancer lung metastases received a single high-dose hypofractionated treatment. In all three cases, tumor shrinkage—including in non-irradiated lesions—was observed within three months after treatment, demonstrating the abscopal effect. Continued shrinkage was observed during follow-up and clearly documented on contrast-enhanced CT/PET-CT. This aligns with previously reported conclusions: lower radiation doses (1.8–2.0 Gy) are insufficient to trigger a strong immune response; moderate doses (8–12 Gy) can enhance immune responses and induce potent anti-tumor effects; while very high doses (15–20 Gy) may reduce the likelihood of the abscopal effect. Hypofractionated radiation can induce strong immunogenicity, thereby facilitating the occurrence of the abscopal effect [21].
The three cases observed in this study did not receive immunotherapy or other pharmacological treatments during their course of therapy. The occurrence of the abscopal effect was entirely attributed to carbon ion radiation, and its potential mechanism is largely consistent with existing research conclusions. However, the limited sample size in this study necessitates further investigation to determine whether the observed effects stem from the unique physical and biological advantages of carbon ions, specific characteristics of the immune system, inherent tumor radiosensitivity, or whether carbon ions are more likely to induce the abscopal effect compared to photons. Additionally, the potential of combining immunotherapy with carbon ion treatment to enhance the abscopal effect requires further exploration. In summary, carbon ions offer advantages such as high linear energy transfer (LET), high relative biological effectiveness (RBE), and the ability to cause complex DNA damage. Compared to conventional radiotherapy, carbon ion therapy has the potential to activate stronger immunogenicity in tumor cells [17]. Further exploration of the mechanisms underlying the carbon ion-induced abscopal effect is of significant importance for developing more effective radiotherapeutic strategies in oncology.
Conflicts of Interest The authors declare no conflicts of interest
Authorship Contribution Statement Tianyan Qin was responsible for data curation, formal analysis, and writing - original draft. Yihe Zhang and Xin Pan were responsible for supervision of data analysis. Yanshan Zhang and Yancheng Ye were responsible for supervision of writing - review & editing. Xin Wang, Yuling Yang, Tong Ma, Caixia Lv, and Pengqing Li were responsible for investigation and data collection.
References
[1] Marcus D, Lieverse R, Klein C, et al. Charged particle and conventional radiotherapy: Current implications as partner for immunotherapy [J].Cancers( Basel),2021,13(6): 1468.DO1: 10.3390/cancers13061468.
[2] 贾蓉,苏锋涛,胡步荣.重离子的辐射生物效应及其在生命科学中的应用[J]. 生物技术通报,2018,34(1):67.78.DOl:10.13560/i.cnki. biotech. bull. 1985.2017-0735. Jia R, Su FT, Hu BR. The biological effects induced by heavyion radiation and its application in life science J. BiotechnolBull,2018,34(1):67-78. DO: 10.13560/j. cnki.biotech. bull. 1985.2017-0735
[3] SCHMID T E, MULTHOFF G. Non-targeted effects of photon and particle irradiation and the interaction with the immune system[J]. Frontiers in Oncology, 2012, 2: 80. DOI: 10.3389/fonc.2012.00080.
[4] KEISARI Y, KELSON I. The potentiation of anti-tumor immunity by tumor abolition with alpha particles, protons, or carbon ion radiation and its enforcement by combination with immunoadjuvants or inhibitors of immune suppressor cells and checkpoint molecules[J]. Cells, 2021, 10(2): 228. DOI: 10.3390/cells10020228.
[5] 高玉婷, 李媛, 金晓东. 碳离子辐射诱导的远隔效应[J]. 生物化学与生物物理进展, 2023, 50(8): 1915-1925. DOI: 10.16476/j.pibb.2022.0371. GAO Y T, LI Y, JIN X D. Abscopal effect induced by carbon ions radiation[J]. Progress in Biochemistry and Biophysics, 2023, 50(8): 1915-1925. DOI: 10.16476/j.pibb.2022.0371.
[6] PARK B, YEE C, LEE K M. The effect of radiation on the immune response to cancers[J]. International Journal of Molecular Sciences, 2014, 15(1): 927-943. DOI: 10.3390/ijms15010927.
[7] MOLE R H. Whole body irradiation: radiobiology or medicine?[J]. British Journal of Radiology, 1953, 26(305): 234-241. DOI: 10.1259/0007-1285-26-305-234.
[8] MATSUBARA S, HORIUCHI J, OKUYAMA T, et al. A case of reticulum cell sarcoma of the skin showing abscopal effect during radiotherapy (author’s transl)[J]. Nihon Igaku Hoshasen Gakkai Zasshi, 1975, 35(10): 860-867.
[9] TOGITANI K, ASAGIRI T, IGUCHI M, et al. Systemic abscopal effect of low-dose radiotherapy (2 Gy × 2) against palatine tonsil follicular lymphoma[J]. Internal Medicine, 2022, 61(20): 3107-3110. DOI: 10.2169/internalmedicine.8968-21.
[10] SASAKI H, KATO J, HORIMOTO K, et al. Delayed-onset abscopal effect after palliative radiotherapy for acral melanoma treated with anti-PD-1 therapy[J]. Journal of Dermatology, 2022, 49(8): e255-e256. DOI: 10.1111/1346-8138.16380.
[11] ZHANG X, ZHANG Y, LIU Y, et al. Stereotactic body radiotherapy-induced abscopal effect twice after pembrolizumab failure in hereditary leiomyomatosis and renal cell carcinoma: a case report with genetic and immunologic analysis[J]. Translational Andrology and Urology, 2021, 10(11): 4304-4312. DOI: 10.21037/tau-21-644.
[12] 丁心静, 卢致辉, 王芬, 等. 远隔效应在非小细胞肺癌免疫治疗时代的研究进展[J]. 肿瘤预防与治疗, 2023, 36(5): 432-439. DOI: 10.3969/j.issn.1674-0904.2023.05.009. DING X J, LU Z H, WANG F, et al. Research progress of abscopal effect in the era of immunotherapy for non-small cell lung cancer[J]. Journal of Cancer Control and Treatment, 2023, 36(5): 432-439. DOI: 10.3969/j.issn.1674-0904.2023.05.009.
[13] 张一贺, 张雁山, 李小军, 等. 碳离子治疗复发胸腺癌产生远隔效应临床观察1例[J]. 中国肿瘤临床, 2021, 48(20): 1075-1076. DOI: 10.12354/j.issn.1000-8179.2021.20211145. ZHANG Y H, ZHANG Y S, LI X J, et al. Clinical observation of distant effect of carbon ion therapy on recurrent thymic carcinoma: a case report[J]. Chinese Journal of Clinical Oncology, 2021, 48(20): 1075-1076. DOI: 10.12354/j.issn.1000-8179.2021.20211145.
[14] 高怡凡, 孙晓蓉, 邢力刚. 放射免疫治疗中远隔效应的作用机制及临床应用[J]. 中华肿瘤防治杂志, 2023, 30(14): 881-886. DOI: 10.16073/j.cnki.cjcpt.2023.14.09. GAO Y F, SUN X R, XING L G. Mechanism and clinical application of abscopal effects in radioimmunotherapy[J]. Chinese Journal of Cancer Prevention and Treatment, 2023, 30(14): 881-886. DOI: 10.16073/j.cnki.cjcpt.2023.14.09.
[15] 徐唐鹏, 胡梦雪, 许斌, 等. 远隔效应的作用机制及临床进展[J]. 肿瘤学杂志, 2019, 25(3): 202-205. DOI: 10.11735/j.issn.1671-170X.2019.03.B005. XU T P, HU M X, XU B, et al. The mechanism and clinical progress on distant effect[J]. Journal of Chinese Oncology, 2019, 25(3): 202-205. DOI: 10.11735/j.issn.1671-170X.2019.03.B005.
[16] GORIN J B, MENAGER J, GOUARD S, et al. Antitumor immunity induced after α irradiation[J]. Neoplasia, 2014, 16(4): 319-328. DOI: 10.1016/j.neo.2014.04.002.
[17] 高玉婷, 李鹏飞, 马国榕, 等. 辐射诱导远隔效应机制的研究进展[J]. 中华放射肿瘤学杂志, 2023, 32(9): 861-865. DOI: 10.3760/cma.j.cn113030-20220822-00285. GAO Y T, LI P F, MA G R, et al. Research progress in the mechanism of abscopal effect induced by radiation[J]. Chinese Journal of Radiation Oncology, 2023, 32(9): 861-865. DOI: 10.3760/cma.j.cn113030-20220822-00285.
[18] DEMARIA S, NG B, DEVITT M L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated[J]. International Journal of Radiation Oncology, Biology, Physics, 2004, 58(3): 862-870. DOI: 10.1016/j.ijrobp.2003.09.012.
[19] 张惠博, 龚虹云, 刘华丽, 等. 远隔效应的研究进展与临床意义[J]. 肿瘤学杂志, 2017, 23(4): 321-326. DOI: 10.11735/j.issn.1671-170X.2017.04.B014. ZHANG H B, GONG H Y, LIU H L, et al. Research progress and clinical significance of abscopal effect of radiotherapy[J]. Journal of Chinese Oncology, 2017, 23(4): 321-326. DOI: 10.11735/j.issn.1671-170X.2017.04.B014.
[20] SHARABI A B, NIRSCHL C J, KOCHEL C M, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen[J]. Cancer Immunology Research, 2015, 3(4): 345-355. DOI: 10.1158/2326-6066.CIR-14-0196.
[21] MORTEZAEE K, PARWAIE W, MOTEVASELI E, et al. Targets for improving tumor response to radiotherapy[J]. International Immunopharmacology, 2019, 76: 105847. DOI: 10.1016/j.intimp.2019.105847.
Preliminary Review: Liu Wenyu
Final Review: Ma Shuqian
