A Dosimetric Comparative Study of Carbon‑Ion Radiotherapy Versus X‑ray Volumetric Modulated Arc Ther
A Dosimetric Comparative Study of Carbon‑Ion Radiotherapy Versus X‑ray Volumetric Modulated Arc Therapy for Stage III Non‑Small‑Cell Lung Cancer
X‑J Li1, C‑R Li2, Y‑C Ye1, Y‑S Zhang1, X‑Q Zong1, CL Feng1
1Heavy Ion Radiotherapy Department, Wuwei Cancer Hospital and Institute, Wuwei Academy of Medical Sciences, Wuwei, Gansu
2Radiotherapy Center, Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, Sichuan, China
Abstract: Background: Compared to photon beam, carbon‑ion radiotherapy (CIRT) has both physical and biological advantages. Aim: To examine whether two‑dimensional (2D) CIRT is dosimetrically superior to photon beam volume‑modulated arc therapy (VMAT) in protecting the normal tissues for stage III non‑small‑cell lung cancer (NSCLC). Subjects and Methods: A retrospective study was conducted. Thirteen patients with stage III NSCLC treated in our center with curative CIRT and a sham photon beam VMAT treatment planning with the same normal tissue dose constraints were included for analysis. Target dose distributions and the homogeneity index (HI) within the planning target volumes were compared. Results: Both CIRT and VMAT plans have good tumor coverage with no significant differences in D98, D95, and D50 of Planning target volume 1 (PTV1) between the two plans. The HIs between the two plans are similar. The HI of PTV2 is superior in the CIRT plan (CIRT vs. VMAT: 0.08 vs. 0.16, P < 0.05). In general, CIRT results in a lower dose of the organ‑at‑risk (OAR) than the photon plans. The V5, V10, V20, V30, V40, and Dmean of the contralateral lung in the CIRT plan are significantly lower than that of the photon VMAT. For the ipsilateral lung, the V5 of CIRT is significantly lower. The CIRT also had significantly lower spinal cord Dmax, esophageal Dmean and V50, V10 and V30 of bone, and V50 of the trachea and bronchial tree. Conclusions: Compared with photon VMAT, 2D‑CIRT using the passive beam scanning technique significantly reduces the radiation dose to the OARs in curative radiotherapy of stage III NSCLC, suggesting a better protection of the normal tissues.
Keywords: Carbon‑ion radiotherapy, dose‑volume parameters, dosimetric comparison, non‑small‑cell lung cancer, volume‑modulated arc therapy (VMAT)
Introduction
According to the International Agency for research on cancer, lung cancer is the second most common malignancy globally after non‑melanoma skin cancer and the first cause of cancer death.[1] In 2018, there were 2.09 million new cases worldwide and 1.76 million lung cancer deaths, representing 11.6% of new cancer diagnoses and 18.4% of cancer mortality, respectively.[1] In China, lung cancer ranks first for cancer incidence and death in 2015, with non‑small‑cell lung cancer (NSCLC) accounting for 80%.[2] About 35% of NSCLC are locally advanced diseases at diagnosis for whom the 5‑year overall survival was a mere 15–25%.[3]
The standard‑of‑care for locally advanced and medically inoperable NSCLC is concurrent chemoradiotherapy plus adjuvant durvalumab with a total radiation dose of 60–70 Gy delivered with a daily dose of 1.8–2 Gy.[4] Multiple dose escalation clinical studies have been conducted with photon beam radiotherapy, trying to improve the treatment outcome of stage III NSCLC. One of these trials, the RTOG 0617, was a phase III randomized trial comparing 60 Gy with 74 Gy. Patients in both arms received conventionally fractionated three‑dimensional conformal radiotherapy (3DCRT) or intensity‑modulated radiotherapy (IMRT) plus concurrent carboplatin and paclitaxel. The median overall survival was 28.7 months for the 60 Gy arm and 20.3 months for the 74 Gy arm (P = 0.0008). An increase in photon radiation dose not only failed to improve survival but also proved to be detrimental to patients.[5] The worse outcome with a higher dose was partly reflected in a higher incidence of severe adverse effects. There were eight treatment‑related deaths in the high‑dose group compared to three in the 60 Gy group. Although the rate of ≥ grade 3 radiation pneumonitis was not statistically different (7% for 74 Gy vs. 4% for 60 Gy, P = 0.25), grade 3 or worse radiation esophagitis was more common in the high‑ versus standard‑dose group (21% vs. 7%, P < 0.001).
Regarding the reason why an increased radiation dose resulted in worse survival in NSCLC patients, an earlier analysis suggested that radiation‑related heart and lung toxicities seemed to be the causes.[6] A later analysis of the impact of different radiotherapy techniques used in the RTOG 0617 trial on survival might shed more lights on the possible mechanisms. The analysis demonstrated that although patients treated with 3DCRT and IMRT did not differ in overall survival, the volume of heart receiving 40 Gy or a higher dose (V40) was significantly associated with OS on adjusted analysis (P < 0.05), and patients with IMRT technique had a significantly lower V40 of heart than the patients with 3DCRT.[7] The lung volume receiving 5 Gy or more dose (V5) was over 50% with both techniques (54.8% in 3DCRT vs. 61.6% in IMRT), indicating that a large volume of lung received a low dose. The high normal tissue radiation dose‑volume in patients treated with photon beam radiotherapy is problematic and one of the outcome‑limiting factors.
Compared to photon beam, carbon ion has both physical and biological advantages. With the Bragg Peak and a sharper penumbra, carbon ion results in a focused dose distribution with a lower dose to normal tissues.[8‑10] Carbon ion also has biological advantages including a higher relative biological effect, a lower oxygen enhancement ratio, and the lack of sublethal damage repair.[11,12] These features make carbon ion a theoretically superior radiotherapy modality compared to photon beams in terms of tumor killing and normal tissue protection.[13‑15] In a phase I clinical study, investigators compared dose‑volume parameters in NSCLC patients treated with CIRT versus patients treated with photon beam stereotactic ablative radiotherapy (SABR).[16] Results showed that CIRT could significantly reduce the dose to the lung, spinal cord, heart, esophagus, and trachea. Our study intends to demonstrate the dosimetric superiority of CIRT compared to photon beam radiotherapy in stage III NSCLC. We compare two‑dimensional‑carbon ion radiotherapy (2D‑CIRT) with photon‑beam volumetric modulated arc therapy (VMAT). Dose‑volumes to normal organs were compared. Hereby, we present our findings.
Subjects and Methods
Patients
Thirteen consecutive stage III NSCLC patients treated with curative CIRT at our center during the period from 2018 to 2021 were included in this analysis. Informed consent was obtained from all individuals included in this study. CIRT was delivered with passive scattering and 2D technique. There were ten right‑lung and three left‑lung cancers. The stages were cT2‑3N1‑2M0 (n = 8) and cT4N2M0 (n = 5) [Table 1]. The study protocol was approved in April 2021 by the institutional ethics review board, with approval number 2021–41.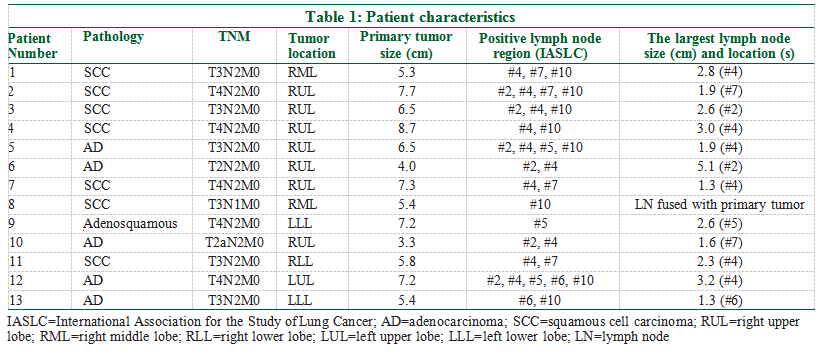 Radiotherapy facilities
Radiotherapy facilities
Patients underwent immobilization with a thermoplastic body mold and then had a CT scanning with a contrast medium by using a large‑bore four‑dimensional CT (4DCT) simulator (Philips Brilliance CT Big Bore Oncology CT) with 2.5‑mm‑thick slices. The scanning range was between the hyoid bone and the superior iliac crest. Images were imported into the ciPlan CIRT planning system (ciPlan, version 1.0, IMP, Lanzhou, China) for target delineation and CIRT planning. A CIRT system designed and manufactured in China was used for patient treatment (HIMM, Lanzhou Kejin Taji Corporation Ltd.). The treatment was delivered with 2–4 portals. Because the gantry was fixed, the patients were irradiated with a supine or prone position, according to the tumor location and its surrounding normal tissues.
CT images of the above 13 patients were retrieved and transferred to the Eclipse 15.5 treatment planning system. A second, sham treatment plan was generated for each patient for photon beam VMAT radiotherapy by using a Vital Beam accelerator equipped with 40 pairs of 0.5 cm multi‑leaf collimators (Varian Medical Systems, Palo Alto, CA).
Target delineation
Gross target volume (GTV) included the primary tumor and clinically positive lymph nodes. Lymph nodes with a largest dimension of 1 cm or more in CT images were considered positive. Clinical target volume (CTV) encompassed the GTV and ipsilateral mediastinal and hilar lymph node regions using international definition.[17] Internal target volume1 (ITV1) was CTV plus the maximum motion projection of the CTV, and ITV2 was GTV plus the maximum motion projection of the GTV. Planning target volume 1 (PTV1) was ITV1 plus 5 mm around all directions, and PTV2 was ITV2 plus 5 mm margin circumferentially. The organ‑at‑risk (OAR), including the lung, spinal cord, esophagus, ribs, clavicles vertebra, trachea, and proximal bronchi, were contoured according to the RTOG 1106 OAR Atlas.[18]
Dose prescription
The same total dose was used for both 2D‑CIRT and photon VMAT. The dose to PTV1 was 48 Gy, and the dose to PTV2 was 24 Gy, with a total PTV2 dose of 72 Gy. Both treatments were delivered with 5 daily fractions per week. However, the dose per fraction was different. The reference point for dose specification in CIRT was the center of PTV. Dose of CIRT was defined as physical dose times the relative biological effect (RBE) and expressed as the equivalent photon dose GyE. For CIRT, 4 Gy (RBE) per fraction was given every day; for photon beam, the dose was meant to be delivered with 2 Gy per fraction. Normal tissue dose constraints were maximum dose (Dmax) 50 Gy for spinal cord and V20 < 35% for total lung volume, among others.
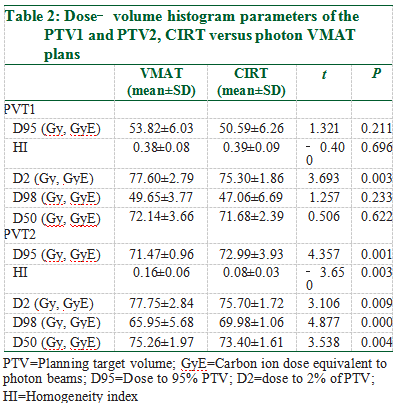
The following dose‑volume histogram parameters were obtained as recommended by the ICRU reports and compared between CIRT and photon VMAT: Target coverage parameters including the dose covering 98% PTV (D98%), D95%, D50% and D2%; the homogeneity index (HI) calculated according to the ICRU definition[19,20]; and the parameters for normal organs including the maximum dose (Dmax), the mean dose (Dmean), the volume of various OAR receiving 5 to 50 Gy or higher dose (V5, V10, V20, V30, V40, and V50). HI = (D2%‑D98%)/D50%. A lower HI indicates a more homogeneous dose distribution.
Statistics
SPSS 22.0 was used for statistical analysis. Paired T test was used for comparison of the mean ± SE of CIRT versus photons. P ˂ 0.05 was considered statistically significant.
Results
Target coverage and target absorbed dose
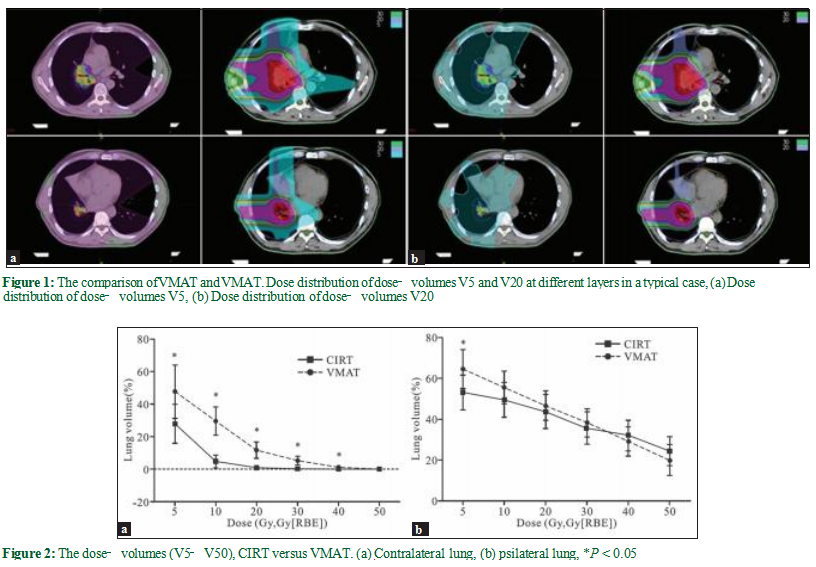
Target coverage was satisfactory for both CIRT and photon VMAT for all 13 patients. Figure 1a and b shows the dose distribution of a typical patient, with 100% prescribed dose covering 95% of the PTV in both CIRT and photon VMAT plans. Table 2 displays the DVH parameters of the PTVs. The D98%, D95%D, 50%, and the HI (CIRT vs. VMAT: 0.39 vs. 0.38, P = 0.696)of the PTV1 were not statistically different between CIRT and the photon VMAT. Compared to VMAT, CIRT had a significantly lower D2% (CIRT vs. VMAT: 75.3 vs. 77.6 Gy, P = 0.003). For PTV2, the D98%, D95% (CIRT vs. VMAT:72.99 GyE vs. 71.47 Gy, P = 0.001), D50%, and the HI (CIRT vs. VMAT: 0.08 vs. 0.16, P = 0.003) were statistically different between CIRT and the photon VMAT, with CIRT achieving a higher absorbed dose and better homogeneity.
Normal tissue doses
In general, the low‑dose volume of the lung was markedly lower in the CIRT than the photon VMAT.
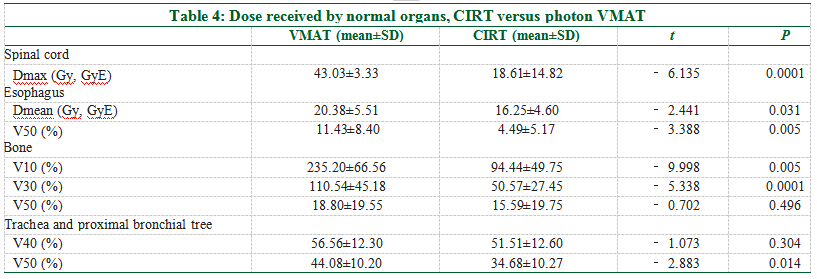
The V5, V10, V20, V30, V40, and Dmean of the contralateral lung in the CIRT plans were significantlly lower than that of the photon VMAT [Figure 2a and Table 3]. The V50 was minimal in both plans with no statistical difference (CIRT vs. VMAT: 0.00 vs. 0.04%, P = 0.082). For the ipsilateral lung, the V5 was significantly lower in the CIRT than the photon plan (CIRT vs. VMAT: 53.00 vs. 64.41%, P = 0.003), while the V10, V20, V30, V40, and Dmean were not statistically different between the two plans [Figure 2b and Table 3].The dose‑volumes of other OARs are listed in Table 4. The Dmax of the spinal cord for CIRT was significantly lower than the photon VMAT (CIRT vs. VMAT: 18.61 vs. 43.03 Gy, P < 0.0001); the Dmean of the esophagus favors CIRT (CIRT vs. VMAT: 16.25 vs. 20.38 Gy, P = 0.031); the V50 of the esophagus was also lower for CIRT (CIRT vs. VMAT: 4.49 vs. 11.43%, P = 0.005); the V10 and V30 of bone and trachea/proximal bronchi were all significantly lower for CIRT, but the V50s were not different between the two plans.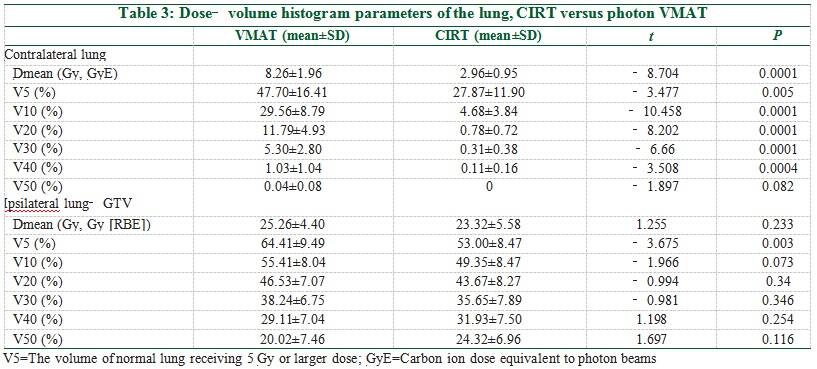
 Discussion
Discussion
This dosimetric analysis demonstrates that both 2D‑CIRT and photon VMAT result in a satisfactory target coverage. Photon VMAT is a high‑end radiation therapy technique with the integration of the image‑guided technique, modulated arc therapy, on‑board image verification, high‑precision treatment table, and a reverse treatment planning system.[21‑24] It therefore represents the highest standard that the photon beam radiotherapy technique can ever reach regarding target coverage. Compared to VMAT, 2D‑CIRT could reach the same target coverage with a better dose homogeneity inside the target. In our patients, there is a dose fall‑off from 72 Gy in PTV1 to 48 Gy in PTV2, and CIRT has a much better dose homogeneity, suggesting a significantly better dose transition.
Lung cancer is surrounded or adjacent to the normal lung, esophagus, heart, spinal cord, ribs, nerves, muscle, and skin. This makes it impossible to avoid the normal tissues during radiotherapy and radiation‑related damages. Despite the unprecedented advancement in the technology of modern radiotherapy, radiation damage is still a critical challenge. In fact, the modern IMRT technique is known for the larger low‑dose volume in normal tissues compared to the conventional 2D photon radiotherapy technique. In a Radiation Therapy Oncology Group (RTOG)‑initiated prospective clinical study, it was shown that radiation pneumonitis, followed by radiation‑related heart toxicities, esophagitis, and myelitis were the dose‑limiting factors in the treatment of NSCLC, and these side effects also have a negative impact on treatment outcome.[25] As multiple clinical trials have shown a high local recurrence rate of up to 30% in stage III NSCLC patients treated with standard concurrent chemoradiotherapy (CCRT) with photon, radiation dose escalation is a reasonable rationale to increase local control.[26,27] However, a higher dose over 60 Gy has failed to improve CCRT treatment outcome in phase III trials, as previously mentioned, likely due to radiotherapy‑related toxicity that accompanied higher‑dose radiotherapy.[5,7] With its unique Bragg Peak, carbon ion is an ideal candidate for dose escalation with normal tissue sparing at the same time. This theoretical advantage has been clinically demonstrated by phase I/II clinical trials conducted by the Japanese National Institute of Radiological Sciences (NIRS).[28] In this trial for stage III NSCLC patients, the normal organ dose constraints were set at 30 GyE for the spinal cord, 50 GyE for esophagus, and 60 GyE for main bronchus. In the phase I part of the trial, only 2 out of 36 patients who received 76 GyE in 16 fractions had grade 3 toxicity. At the phase II part of the trial where all patients received CIRT with 72 GyE, no grade 3 or greater toxicity occurred. The 2‑year local control rate and overall survival rate were 93.1% and 51.9%, respectively. For the sub‑group of patients with cT3‑4N0 patients, the outcomes were better, with a 2‑year local control rate of 100% and overall survival of 69.3%.
Radiation pneumonitis is one of the most significant toxicities associated with CCRT in lung cancer patients treated with photon beam. Numerous studies have tried to investigate this potentially lethal adverse event from different aspects. One major effort has been to define the dose‑volume histogram (DVH) parameters which can predict the risk and severity of radiation pneumonitis. In a typical study by Tsujino et al., V20 was found to be a useful predictor.[29] For patients with V20 <20%, 21–25%, 26–30%, and >31%, the incidence of ≥grade 2 radiation pneumonitis in 6 months was 8.7%, 18.3%, 51%, and 85%, respectively. These results show that an increase of a few percentages of V20 would lead to a markedly higher risk of radiation pneumonitis.
In our present analysis, we demonstrate that there is a 11.01% and 2.86% absolute decrease of V20 in the contralateral and ipsilateral lung for CIRT compared with photon VMAT plans [Table 3]; the V5 was 19.83% and 11.41% lower in the CIRT plans. The mean lung dose (MLD) of the contralateral lung was 5.3 Gy lower in the CIRT than the photon plans [Table 3]. There were reports showing a better predictive value of V5, V20, and MLD for radiation pneumonitis than other parameters.[30‑32] Marks et al. proposed a mathematic model based on MLD to predict the risk of radiation pneumonitis.[32] In our analysis, MLD was 8.26 ± 1.96 Gy with photon VMAT versus 2.96 ± 0.95 Gy with CIRT for the contralateral lung, and 25.26 ± 4.40 Gy with VMAT vs. 23.32 ± 5.58 Gy with CIRT for the ipsilateral lung. By fitting this model, the probability of radiation pneumonitis was 14.70% for CIRT versus 9.84% for photon VMAT; CIRT would result in a lower risk of radiation pneumonitis than the photon VMAT.
CIRT also reduces the radiation dose in other OARs. It has been reported that both Dmean and V50 of the esophagus can be used to predict the risk of radiation esophagitis.[33] Our analysis shows that CIRT results in a 20% reduction of Dmean and 60.7% lower V50 esophageal dose. In addition, CIRT plans show a lower dose‑volume of the spinal cord and bone (V10 and V30) and the trachea and peripheral bronchial tree (V50).
In this analysis, CIRT plans did not show an advantage of the V40, V50, and MLD in the ipsilateral lung; it also fails to fare better than the photons in the V50 of bone and V40 of the trachea and peripheral bronchial tree. This is the combined results of the passive scattering technique used in the present 2D‑CIRT and the compensator required for 2D‑CIRT. This is exactly the limitation of our analysis. Our CIRT facility will soon be upgraded to the scanning beam technique. This limitation will be abolished when the more advanced scanning beam technique is put to clinical use, and the dose distribution advantage will be further enhanced.
While this dosimetry study shows promise for CIRT in stage III NSCLC, further clinical studies are warranted to confirm whether the dosimetric advantages translate to reduced acute and late toxicities. Assessing acute toxicities will be important to ensure the safety of CIRT delivery. Additionally, longer‑term follow‑up on tumor control and late toxicities in a phase I/II trial comparing CIRT to photon therapy will help quantify clinical benefits and risks prior to a definitive phase III study. With rigorous toxicity and efficacy data from early phase trials, we can determine if the dosimetric profile of CIRT merits evaluation in a large randomized study versus the current standard photon therapy for locally advanced NSCLC.
Conclusion
The advantages of 2D‑CIRT for NSCLC patients, compared to photon VMAT, include a higher dose homogeneity in the target and lower normal tissue dose‑volumes and the subsequent lower risk of toxicity. It appears to be a safe and effective treatment option for stage III NSCLC.
Informed consent
Informed consent was obtained from all individuals included in this study.
Ethical approval
The study protocol was approved in April 2021 by the institutional ethics review board, with approval number: 2021‑41.
Author contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Financial support and sponsorship
This study was supported by The Innovation Base and Talent Program of Science and Technology Department of Gansu Province, China, No. 21JR7RH896, and The Longyuan Young Talents Project in Gansu Province, China. The funding organization(s) plays no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision in to submit the report for publication.
Conflicts of interest
Authors state no conflict of interest.
References
1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394‑424.
2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115‑32.
3.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta‑analysis of concomitant versus sequential radiochemotherapy in locally advanced non‑small‑cell lung cancer. J Clin Oncol 2010;28:2181‑90.
4.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‑small‑cell lung cancer. N Engl J Med 2017;377:1919‑29.
5.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard‑dose versus high‑dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‑small‑cell lung cancer (RTOG 0617):A randomised, two‑by‑two factorial phase 3 study. Lancet Oncol 2015;16:187‑99.
6.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys 2012;82:1042‑4.
7.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of intensity‑modulated radiation therapy technique for locally advanced non‑small‑cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017;35:56‑62.
8.Kanai T, Furusawa Y, Fukutsu K, Itsukaichi H, Eguchi‑Kasai K, Ohara H. Irradiation of mixed beam and design of spread‑out Bragg peak for heavy‑ion radiotherapy. Radiat Res 1997;147:78‑85.
9.Schulz‑Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 2007;25:953‑64.
10.Wang L, Hu J, Liu X, Wang W, Kong L, Lu JJ. Intensity‑modulated carbon‑ion radiation therapy versus intensity‑modulated photon‑based radiation therapy in locally recurrent nasopharyngeal carcinoma: A dosimetric comparison. Cancer Manag Res 2019;11:7767‑77.
11.Chatzipapas KP, Papadimitroulas P, Emfietzoglou D, Kalospyros SA, Hada M, Georgakilas AG, et al. Ionizing radiation and complex DNA damage: Quantifying the radiobiological damage using monte carlo simulations. Cancers (Basel) 2020;12:799.
12.Buglewicz DJ, Walsh KD, Hirakawa H, Kitamura H, Fujimori A, Kato TA. Biological effects of monoenergetic carbon ions and their associated secondary particles. Front Oncol 2022;12:788293.
13.Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol 2012;42:670‑85.
14.Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol 2010;7:37‑43.
15.Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi‑Kasai K, Ohara H, et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3) He‑ , (12) C‑ and (20) Ne‑ion beams. Radiat Res 2000;154:485‑96.
16.Ebara T, Shimada H, Kawamura H, Shirai K, Saito J, Kawashima M, et al. Dosimetric analysis between carbon ion radiotherapy and stereotactic body radiotherapy in stage I lung cancer. Anticancer Res 2014;34:5099‑104.
17.Goldstraw P, Chansky K, Crowley J, Rami‑Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39‑51.
18.Kong FM, Ritter T, Quint DJ, Senan S, Gaspar LE, Komaki RU, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: Alung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus.Int J Radiat Oncol Biol Phys 2011;81:1442‑57.
19.Hodapp N. [The ICRU Report 83: Prescribing, recording and reporting photon‑beam intensity‑modulated radiation therapy (IMRT)]. Strahlenther Onkol 2012;188:97‑9.
20.1.Special Considerations Regarding Absorbed‑Dose and Dose‑Volume Prescribing and Reporting in IMRT. J ICRU 2010;10:27‑40.
21.Akcay M, Etiz D, Duruer K, Bozdogan O, Ozen A. Dosimetric comparison of single‑arc/partial‑arc volumetric modulated arc therapy and intensity‑modulated radiotherapy for peripheral and central lung cancer. J Cancer Res Ther 2021;17:80‑7.
22.Ren W, Sun C, Lu N, Xu Y, Han F, Liu YP, et al. Dosimetric comparison of intensity‑modulated radiotherapy and volumetric‑modulated arc radiotherapy in patients with prostate cancer: A meta‑analysis. J Appl Clin Med Phys 2016;17:254‑62.
23.Yu CX, Tang G. Intensity‑modulated arc therapy: Principles, technologies and clinical implementation. Phys Med Biol 2011;56:R31‑54.
24.Hunte SO, Clark CH, Zyuzikov N, Nisbet A. Volumetric modulated arc therapy (VMAT): A review of clinical outcomes‑what is the clinical evidence for the most effective implementation? Br J Radiol 2022;95:20201289.
25.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose‑volume histogram analysis for pneumonitis after 3D treatment for non‑small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323‑9.
26.Curran WJ, Jr., Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non‑small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452‑60.
27.Rodriguez De Dios N, Navarro‑Martin A, Cigarral C, Chicas‑Sett R, Garcia R, Garcia V, et al. GOECP/SEOR radiotheraphy guidelines for non‑small‑cell lung cancer. World J Clin Oncol 2022;13:237‑66.
28.Takahashi W, Nakajima M, Yamamoto N, Yamashita H, Nakagawa K, Miyamoto T, etal. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non‑small cell lung cancer (NSCLC). Cancer 2015;121:1321‑7.
29.Tsujino K, Hirota S, Endo M, Obayashi K, Kotani Y, Satouchi M, et al. Predictive value of dose‑volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2003;55:110‑5.
30.Wang S, LiaoZ, WeiX, LiuHH, Tucker SL, Hu CS, et al. Analysis of clinical and dosimetric factors associated with treatment‑related pneumonitis (TRP) in patients with non‑small‑cell lung cancer (NSCLC) treated with concurrent chemotherapy and three‑dimensional conformal radiotherapy (3D‑CRT). Int J Radiat Oncol Biol Phys 2006;66:1399‑407.
31.Kong FM, Hayman JA, Griffith KA, Kalemkerian GP, Arenberg D, Lyons S, et al. Final toxicity results of a radiation‑dose escalation study in patients with non‑small‑cell lung cancer (NSCLC): Predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys 2006;65:1075‑86.
32.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose‑volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76 (3 Suppl):S70‑6.
33.Yu Y, Zheng H, Liu L, Li H, Zheng Q, Wang Z, et al. Predicting severe radiation esophagitis in patients with locally advanced esophageal squamous cell carcinoma receiving definitive chemoradiotherapy: Construction and validation of a model based in the clinical and dosimetric parameters as well as inflammatory indexes. Front Oncol 2021;11:687035.
Preliminary Review: Zhang Lihong
Final Review: Zhang Jie
