A Retrospective Study of Chemotherapy and 3D-Image-Guided Afterloading Intracavitary Radiotherapy in
A Retrospective Study of Chemotherapy and 3D-Image-Guided Afterloading Intracavitary Radiotherapy in Locally Advanced Cervical Cancer
Xiaojun Li ,1 Cunlian An ,2 Chunlan Feng,2 Jieren Sun,1 Huixiang Lu,1 XiaodongYang,1 Kaiping Wang,1 and Ruimei Wang2
1Heavy Ion Radiotherapy Department, Wuwei Cancer Hospital and Institute, Wuwei Academy of Medical Sciences, Gansu, China 733000
2Department of Gynecology and Oncology, Wuwei Cancer Hospital, Gansu, China 733000 Correspondence should be addressed to Cunlian An; clianan@hbut.edu.cn
Received 28 August 2022; Accepted 12 September 2022; Published 30 September 2022
Academic Editor: Zhongjie Shi
Copyright © 2022 Xiaojun Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aim. To investigate the value of neoadjuvant chemotherapy combined with 3D-image-guided afterloading intracavitary radiotherapy in locally advanced cervical cancer (LACC). Methods. Patients with cervical cancer admitted to our hospital from January 1, 2020 to January 1, 2021 were retrieved and analyzed. Cases treated with neoadjuvant chemotherapy and 3D-image-guided afterloading intracavitary radiotherapy were assigned into the observation group (OG), while cases with neoadjuvant chemotherapy alone were assigned into the control group (CG). The short-term effects were determined by RECIST 1.1. Total effective rate (TR) = complete remission (CR) + partial remission (PR). The serum levels of squamous epithelial cell carcinoma antigen (SCC-Ag), glycoantigen 125 (CA125), carcinoembryonic antigen (CEA), and vascular endothelial growth factor (VEGF) were assessed. In view of the difference between tumor markers and diameters before and after treatment, the correlation between them was analyzed by Pearson test. The adverse events were compared, and the amount of operative bleeding and operation time were evaluated. Cox regression analysis was conducted to assess the influencing factors of 1-year disease-free survival time. Results. Sixty-seven patients were retrieved, including 30 cases in the OG and 37 cases in the CG. There were no significant differences in age, pathological type, tumor size, FIGO stage, past medical history, or smoking history between the two groups (P >0.05). The TR of patients in the OG was higher than that in the CG (P <0.05). The SCC-Ag, CA125, CEA, and VEGF levels in the OG decreased markedly after treatment (P <0.001). The difference in SCC-Ag, CA125, CEA, and VEGF was positively correlated with the difference in tumor diameter before and after treatment (P <0.05). The incidence of adverse events revealed no obvious difference between the OG and CG (P >0.05). Cox regression analysis showed that FIGO stage and treatment regimens were independent prognostic factors for 1-year disease-free survival (P <0.05). Conclusion. Neoadjuvant chemotherapy combined with 3D-image-guided afterloading intracavitary radiotherapy can improve the TR rate and 1-year disease-free survival of LACC patients without increasing the incidence of adverse events.
1. Introduction
Globally, cervical cancer (CC) ranks among malignancies with the highest number of new cases and deaths, posing a serious threat to the health of women [1]. In China, CC screening still needs to be popularized due to uneven regional healthcare development, and many patients are already in the stage of locally advanced cervical cancer (LACC) at initial diagnosis [2, 3], who are unable to be treated solely by surgeries [4, 5]. The 5-year survival rates of CC patients in stage IB1 and IIA1 are 80%-90% and 79.7%, respectively, while those of stage IB2 and IIA2 decreased to 50%-60% [6, 7].
Radical concurrent radiotherapy is the standard of care for advanced CC with NCCN guideline class 1 evidence, but the optimal treatment regimen for LACC is currently highly controversial, and there is no consensus worldwide [8]. The standard treatment recommended in the United States and Canada is concurrent radiotherapy, while countries in Europe, Asia, and Latin America use neoadjuvant chemotherapy followed by surgery as first-line treatment [9]. LACC patients are difficult to cure and have a poor prognosis due to the large localized extent of the tumor and the high risk factors [10]. For LACC with tumor diameter ≥ 4cm, it is not easily controlled by surgical treatment alone and is prone to distant metastases and lymph node metastases after surgery [11]. Currently, the preoperative adjuvant treatment options mainly include neoadjuvant chemotherapy and radiotherapy. Radiotherapy is a local treatment, while chemotherapy can treat distant metastases and lymph node metastases while reducing the tumor [12]. Despite the international controversy regarding preoperative adjuvant therapy for LACC, preoperative adjuvant radiotherapy, neoadjuvant chemotherapy, or their combination are still popular in developing countries by reducing tumor volume, improving the tissue environment around the uterus, and facilitating surgical operation. Also, they can reduce the difficulty of surgery, improve the surgical resection rate of patients, and control tumors effectively [13]. Research has proven that combining radiotherapy with chemotherapy is an even more effective way to improve the local control rate of advanced CC [14]. However, radiotherapy alone can increase drug resistance and lead to many side effects.
To reduce the side effects of radiotherapy toxicity and to ensure efficient and sustainable treatment, it has been a hot topic of research in the gynecologic oncology field. In this study, neoadjuvant chemotherapy combined with 3D-image-guided afterloading intracavitary radiotherapy was offered to LACC patients prior to radical hysterectomy to observe the short-term clinical impact and outcome as well as adverse events, so as to evaluate the clinical significance of this regimen in future treatment.
2. Methods and Materials
CC patients treated at our hospital from January 1, 2020 to January 1, 2021 were analyzed retrospectively. Cases treated with neoadjuvant chemotherapy and 3D-image-guided afterloading intracavitary radiotherapy were assigned into the observation group (OG), while cases with neoadjuvant chemotherapy alone were enrolled into the control group (CG). All patients received radical CC surgery after treat- ment. The research was conducted with the approval of the medical ethics committee of Wuwei Cancer Hospital and Institute.
The inclusion criteria were cases confirmed through his- topathology. The gynecologic examinations were performed by two gynecologic oncologists of associate chief physician or above and diagnosed according to the FIGO stage IB2 and IIA2 (FIGO staging 2009) [15]. Patients should not receive targeted treatment before this research. Patients’ clinical data were complete. All of them were informed and signed an informed consent form. The exclusion criteria were as follows: cases with serious complications or underlying diseases that could not tolerate the treatment plan; cases complicated with other malignancies; surgical history of cer- vical disease; history of radiotherapy, chemotherapy, or anti- tumor therapy; infectious or metabolic diseases; abnormal blood clotting function; cognitive impairment or mental ill- ness; patients with allergic symptoms of chemotherapeutic drugs; and patients during lactation or pregnancy.
2.1. Treatment Regimens. Patients received neoadjuvant chemotherapy with paclitaxel plus platinum, which was sensitiveto CC. Specifically, paclitaxel 135-175mg/m2 was given intravenously on day 1, and cisplatin 50-75mg/m2 was given on days 1 to 3. The chemotherapy was administered at 3- week intervals for 2 cycles, during which symptomatic treat- ments such as hydration and antiemetic were routinely used. The 3D-afterloading intracavitary radiation therapy with 5.5-6Gy each time was performed twice and completed within 1 week [16]. Radical hysterectomy and pelvic lymph node dissection was performed 2 weeks after adjuvant therapy.
2.2. Outcome Determinations. The main outcomes include: the near-term outcomes were compared by the Response evaluation criteria in solid tumors version 1.1 (RECIST 1.1) [17]. Total response rate (TR) = complete response( CR) + partial response (PR). The squamous cell carcinoma antigen (SCC-Ag), carbohydrate antigen (CA125), and car- cinoembryonic antigen (CEA) of squamous cell carcinoma before and after treatment were tested by chemilumines- cence method [18], and the level of serum vascular endothe- lial growth factor (VEGF) was determined by enzyme linked immunosorbent assay (ELISA). The correlation between tumor markers and diameter changes was assessed by Pear- son’s test according to the diference between patients before and after treatment.
The secondary outcomes include: the clinical characteris-tics and adverse events of both groups were compared. The amount of intraoperative bleeding and operation time were assessed. Cox regression analysis was conducted to assess the influencing factors of 1-year disease-free survival time.
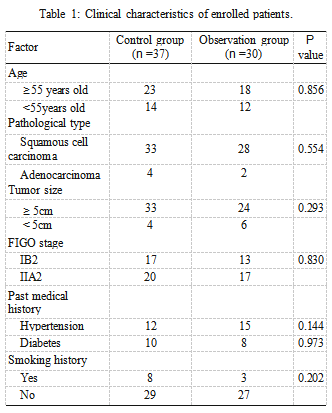
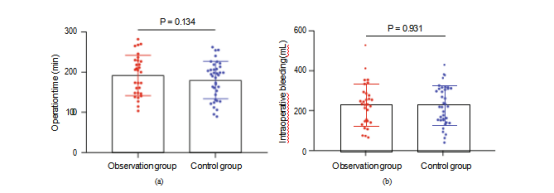
Figure 1: Blood loss and operation time during operation. (a) Diference of operation time between groups. (b) Diference of intraoperative blood loss between groups.
Table 2: Comparison of near-term efficacy [n (%)].
Figure 2: Comparison of serum tumor markers and VEGF levels inpatients before and after treatment. (a) Comparison of serum SCC-Ag levels between groups before and after treatment. (b) Comparison of serum CA125 levels between groups before and after treatment. (c) Comparison of serum CEA levels between groups before and after treatment. (d) Changes of serum VEGF levels before and after treatment.
Table 3: Diference of various indexes before and after treatment.
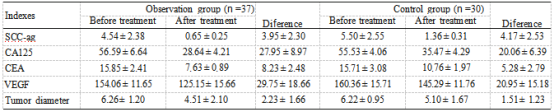
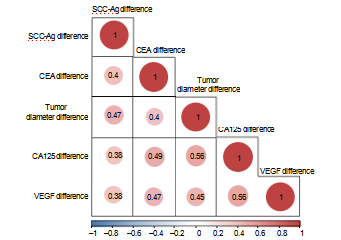
Figure 3: Correlation between tumor diameter, markers, and VEGF. Note: red indicates positive correlation,
and blue indicates negative correlation.
Table 4: Adverse events of patients.
Figure 4: Analysis of age, FIGO stage, treatment plan, and disease-free survival of patients. (a) Analysis of age and disease-free survival of patients. (b) Analysis ofFIGO staging and disease-free survival of patients. (c) Analysis of treatment plans and disease-free survival time of patients.
Table 5: Analysis of risk factors for disease-free survival time.
2.3. Statistical Analysis. Data were analyzed through SPSS 20.0 (IBM Corp., Armonk, N.Y., USA). The enumeration data were expressed as n (%) and analyzed using the chi- squared test, and the measurement data were shown as mean ± standard deviation (SD) and evaluated by indepen- dent t-test. The association between tumor markers and diameter was assessed via Pearson test. Patients’ disease- free survivals were plotted using the Kaplan-Meier survival curves, and then analyzed through log-rank test. The prog- nostic factor afecting patients’ disease-free survival time was assessed through Cox regression. A two-tailed p value <0.05 indicated statistical diference.
3. Results
3.1. Comparison of Clinical Characteristics. Sixty-seven CC patients were retrieved, including 30 cases in the OG and 37 cases in the CG. There were no statistical diferences in age, pathological type, tumor size, FIGO stage, past medical history, or smoking history between the two groups (P >0.05, Table 1). There was no marked diference in oper- ation time and intraoperative blood loss between groups (P >0.05, Figure 1).
3.2. Comparison of Near-Term Efficacy after Radiotherapy and Chemotherapy. There was significantly higher TR of patients in the OG than that in the CG (P <0.05, Table 2).
3.3. Changes of Tumor Markers and VEGF Expression before and after Treatment. The changes of serum tumor markers and VEGF expression were compared after radiotherapy and chemotherapy before operation. The SCC-Ag, CA125, CEA, and VEGF levels in serum after treatment were lower than those before treatment (P <0.001). After treatment, the levels of SCC-Ag, CA125, CEA, and VEGF in the OG were significantly lower than those in the CG (P <0.001, Figure 2).
3.4. Correlation between Tumor Markers, VAGF, and Diameter. We performed a correlation analysis based on the diferences of indicators before and after treatment (Table 3). The diferences of SCC-Ag, CA125, CEA, and VEGF before and after treatment were positively correlated with those of tumor diameter (P <0.05, Figure 3).
3.5. Comparison of Adverse Events in Patients. There was no obvious diference in the incidence of adverse events between groups (P >0.05, Table 4).
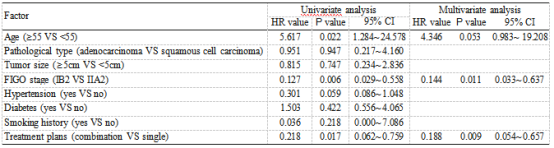
3.6. Analysis of Prognostic Factors of Disease-Free Survival Time. The 1-year disease-free survival rate of 67 patients was 74.62%. Subsequently, we analyzed the clinical data of patients using univariate analysis and found that age, FIGO stage, and treatment regimens were prognostic fac- tors afecting disease-free survival (Figure 4). Further anal- ysis revealed that FIGO staging and treatment regimens were independently tied to patients’ disease-free survival (P <0.05, Table 5).
4. Discussion
CC is a malignancy with high incidence in female patients. LACC accounts for a relatively large proportion among CC, and the 5-year survival rate is about 60% [19]. The tumor diameter of LACC patients is relatively large, which increases the difficulty of operation to a certain extent. The incidence of postoperative complications, metastasis, and recurrence rate are high, and the prognosis is not ideal [20]. Not only that, clinical treatment is difficult and more controversial. Although concurrent chemoradiation is con- sidered an international standard treatment option, there are still many problems and limitations [21]. The number of patients treated tends to be younger, and radical surgery after preoperative adjuvant treatment is more in line with clinical needs. Neoadjuvant chemotherapy and 3D-image- guided afterloading intracavitary radiotherapy combines the advantages of precision radiotherapy and chemotherapy, so that CC with large tumor size can be well controlled, cre- ating favorable conditions for surgical resection, and reduc- ing surgical risks and complications. It improves the efect of treatment and quality of life of patients efectively [22]. Nev- ertheless, there are few studies on whether there is a difer- ence in the efficacy between combination therapy and neoadjuvant chemotherapy alone in LACC treatment [23].
In the present study, we analyzed the efficacy of the two regimens in LACC patients. In our study, we found no marked efect of the two regimens on overall outcomes and adverse events. But our further analysis revealed that the TR rate of patients in the OG was higher than that of those in the CG. Previously, research found that 3D-image-guided afterloading intracavitary radiotherapy combined with che- motherapy improved the treatment outcome of advanced CC [24]. This is due to the fact that 3D conformal HDR brachytherapy can calculate the radiation dose received by the target area and surrounding normal organ tissues more accurately, which is conducive to the development of reason- able individualized treatment plans [25]. Neoadjuvant che- motherapy and radiotherapy have synergistic efects, acting on diferent cell cycles, respectively. Chemotherapy synchro- nizes cancer cells with radiotherapy-sensitive cycles, increases radiotherapy sensitivity while shrinking tumor cells, accelerates the apoptotic process of cancer cells, and reduces the chance of CC metastasis, thus, improving the histopathology [26]. Radiotherapy shrinks the local mass and leads to narrowing and occlusion of some capillaries and lymphatic vessels in the pelvis, which facilitates surgical operations and reduces the difficulty of surgery, thus, improving the efficiency of surgery [27]. We also found no diference in intraoperative bleeding and operative time during surgery. It is theoretically believed that combined treat- ment can reduce the local tumor volume and improve the parametrial tissue gap, which in turn reduces the difficulty of surgery. It showed that the combined treatment did not reduce the difficulty of the procedure, and we believe that the physiology of patients was diminished after the com- bined treatment. In addition, after the combined treatment, the reactive adhesion of lymphoid tissue and the para- uterine tissue fibrosis increased, thus, increasing the diffi - culty of the operation.
Currently, tumor markers such as SCC-Ag, CA125, CEA, and VEGF are of clinical value in CC diagnosis and treatment [28]. SCC-Ag is a relevant antigen reflecting the proliferation of squamous epithelial cells [29]. CA125 is one of the specific tumor markers, mostly found in adult pleura, endometrium, fallopian tube endothelium, and endocervical lining, and its expression is relevant to the tumor load in patients [30]. CEA may reflect the risk of tumor cell infiltration [18]. VEGF is an essential vascular endothelial growth factor for distant metastasis and tumor recurrence [31]. We found the SCC-Ag, CA125, CEA, and VEGF expression in LACC patients decreased after treat- ment. Besides, we confirmed a positive correlation between the diference of SCC-Ag, CA125, CEA, VEGF, and tumor diameter, which indicates that SCC-Ag, CA125, CEA, and VEGF are relevant to tumor growth. It is suggested that joint observation of changes in the levels of these markers may have vital monitoring value for assessing the disease progres- sion and treatment efficacy.
At the end of the research, we measured patients’ disease-free survival time. Cox regression analysis revealed that FIGO staging and treatment regimens were relevant to their disease-free survival. Earlier studies have shown that patients with higher FIGO stage have shorter disease-free survival time, which is consistent with our findings [32]. We first found that neoadjuvant chemotherapy combined with 3D-image-guided afterloading intracavitary radiother- apy was efective in improving the short-term disease-free survival of LACC patients. We believe this is due to the fact that preoperative radiotherapy shrinks the local mass. More- over, preoperative radiotherapy can reduce local cervical tumors in varying degrees, which can not only eliminate tumor cells or reduce their activity, block tumor vessels, improve para-uterine infiltration, increase surgical resection rate but also reduce intraoperative dissemination and improve survival rate.
We found that neoadjuvant chemotherapy combined with 3D-image-guided afterloading intracavitary radiother- apy can increase the TR rate of LACC patients and improve their short-term disease-free survival. Nevertheless, there are still some limitations. First of all, the study period is relatively short. We were only able to count the disease-free survival time of patients for one year, and the effect of combined therapy on long-term overall survival and disease-free survival needs further study. Second, we only collected a relatively small number of patients for this study. Finally, this was a retrospective study, the results of which might be biased. We hope to continue to follow patients in subsequent studies and retrieve more patients to confirm our findings. It might be more intriguing to consider the combined therapy in other complicated cases, such as infection, hypoxia, fulminant hepatitis, or wound healing problems [33–39].
To sum up, neoadjuvant chemotherapy combined with 3D-image-guided afterloading intracavitary radiotherapy in LACC patients improves the TR rates and 1-year disease-free survival and does not increase the incidence of adverse events.
Data Availability
The data used to support the findings of this study are avail- able from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was supported by the second batch of municipal science and technology plan projects in Wuwei City in 2020 and 2021(WW2002079 and WW2101137).
References
[1] F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, and A. Jemal, “Global cancer statistics 2018: GLOBOCANesti- mates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA: a Cancer Journal for Clinicians, vol. 68, no. 6, pp. 394–424, 2018.
[2] A. Gadducci and S. Cosio, “Neoadjuvant chemotherapy in locally advanced cervical cancer: review of the literature and perspectives of clinical research,” Anticancer Research, vol. 40, no. 9, pp. 4819–4828, 2020.
[3] H. B. Musunuru, P. M. Pifer, P. Mohindra, K. Albuquerque, and S. Beriwal, “Advances in management of locally advanced cervical cancer,” The Indian Journal of Medical Research, vol. 154, no. 2, pp. 248–261, 2021.
[4] S. Chopra, M. Gupta, A. Mathew et al., “Locally advanced cer- vical cancer: a study of 5-year outcomes,” Indian Journal of Cancer, vol. 55, no. 1, pp. 45–49, 2018.
[5] R. Benson, S. Pathy, L. Kumar, S. Mathur, V. Dadhwal, and B. K. Mohanti, “Locally advanced cervical cancer - neoadju-
vant chemotherapy followed by concurrent chemoradiation and targeted therapy as maintenance: a phase II study,” Jour- nal of Cancer Research and Therapeutics, vol. 15, no. 6, pp. 1359–1364, 2019.
[6] T. Shu, B. Li, D. Zhao,Y. T. Wang, and S. H. Liu, “Open nerve- plane sparing radical hysterectomy in locally advanced cervical cancer: evaluation on efficacy and long-term survival out- comes,” Zhonghua Fu Chan Ke Za Zhi, vol. 56, no. 1, pp. 43– 51, 2021.
[7] K. G. G. van Kol, R. M. F. Ebisch,J. M. J. Piek, P. L. M. Zuster- zeel, T. F. M. Vergeldt,and R. L. M. Bekkers, “Salvage surgery for patients with residual disease after chemoradiation therapy for locally advanced cervical cancer: a systematic review on indication, complications, and survival,” Acta Obstetricia et Gynecologica Scandinavica, vol. 100, no. 7, pp. 1176–1185, 2021.
[8] V. A. Fabri, A. C. M. Queiroz, H. Mantoan et al., “The impact of addition of consolidation chemotherapy to standard cisplatin-based chemoradiotherapy in uterine cervical cancer: matter of distant relapse,” Journal of Oncology, vol. 2019, Arti- cle ID 1217838, 9 pages, 2019.
[9] W. Zou, Y. Han, Y. Zhang et al., “Neoadjuvant chemotherapy plus surgery versus concurrent chemoradiotherapy in stage IB2-IIB cervical cancer: a systematic review and meta-analy- sis,” PLoS One, vol. 14, no. 11, article e0225264, 2019.
[10] M. Toure, A. T. Bambara, K. K. Y. Kouassi etal., “Level of con-cordance of pre-, intra-, and postoperative staging in cervical cancers (TREYA study),” Journal of Oncology, vol. 2017, Article ID 8201462, 5 pages, 2017.
[11] N. Sakuragi, M. Kaneuchi, T. Kato et al., “Tailored radical hys-terectomy for locally advanced cervical cancer,” International Journal of Gynecological Cancer, vol. 30, no. 8, pp. 1136–1142, 2020.
[12] X. Tian, F. Yang, F. Li et al., “A comparison of different schemes of neoadjuvant chemotherapy followed by concurrent chemotherapy and radiotherapy for locally advanced cervical cancer: a retrospective study,” Cancer Management and Research, vol. 13, pp. 8307–8316, 2021.
[13] L. C. Wei, X. Li, Y. Zhang et al., “Individualized pelvic lymph- adenectomy should follow neoadjuvant concurrent chemora- diotherapy for locally advanced cervical cancer,” Medicine, vol. 97, no. 14, article e0331, 2018.
[14] T. Asakij,J. Khunnarong, S. Tangjitgamoletal., “Salvage treat- ment and outcomes of locally advanced cervical cancer after failed concurrent chemoradiation with or without adjuvant chemotherapy: post hoc data analysis from the ACTLACC trial,” Asian Pacific Journal of Cancer Prevention, vol. 23, no. 7, pp. 2263–2269, 2022.
[15] A. Mohamud, C. Hogdall, and T. Schnack, “Prognostic value of the 2018 FIGO staging system for cervical cancer,” Gyneco- logic Oncology, vol. 165, no. 3, pp. 506–513, 2022.
[16] J. Itami, N. Murakami, M. Watanabe et al., “Combined inter- stitialand intracavitary high-dose rate brachytherapy ofcervi- cal cancer,” Frontiers in Oncology, vol. 11, p. 809825, 2022.
[17] R. L. Wahl, H. Jacene, Y. Kasamon, and M. A. Lodge, “From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors,” Journal of Nuclear Medicine, vol. 50, Suppl 1, pp. 122S–150S, 2009.
[18] L. Gao,J. Lv, L. Hou, Y. Yuan, and Q. Wan, “Clinical efects of Chinese herbal decoction combined with basic chemoradio- therapy and nursing intervention in the treatment of cervical cancer and the efect on serum CEA, CA125, and TNF-α levels,” Evidence-based Complementary and Alternative Medi- cine, vol. 2021, Article ID 1446864, 7 pages, 2021.
[19] J. Ge,J. Sun,J. Li,Q. Zhang,X. Lv, and B. Chen, “Operation for locally advanced cervical cancer after concurrent chemoradio- therapy,” International Journal of Clinical Oncology, vol. 25, no. 5, pp. 948–954, 2020.
[20] F. Landoni, A. Colombo, R. Milani, F. Placa, V. Zanagnolo, and C. Mangioni, “Randomized study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update,” Journal of Gynecologic Oncology, vol. 28, no. 3, article e34, 2017.
[21] S.Tangjitgamol, K. Katanyoo, M. Laopaiboon, P. Lumbiganon, S. Manusirivithaya, and B. Supawattanabodee, “Adjuvant chemotherapy after concur- rent chemoradiation for locally advanced cervical cancer,” Cochrane Database of Systematic Reviews, vol.12, article CD010401, 2014.
[22] A. Mousavi, M. Modarres Gilani, S. Akhavan, S. Sheikh Hasani, A. Alipour, and H. Gholami, “The outcome of locally advanced cervical cancer inpatients treated with neoadjuvant chemotherapy followed by radical hysterectomy and primary surgery,” The Iranian Journal of Medical Sciences, vol. 46, no. 5, pp. 355–363, 2021.
[23] T. Kokabu, K. Masui, Y. Tarumi et al., “3D-image-guided multi-catheter interstitial brachytherapy for bulky and high- risk stage IIB-IVB cervical cancer,” Cancers, vol. 14, no. 5, p. 1257, 2022.
[24] P. Naga Ch, L. Gurram, S. Chopra, and U. Mahantshetty, “The management of locally advanced cervical cancer,” Current Opinion in Oncology, vol. 30, no. 5, pp. 323–329, 2018.
[25] N. B. Chen, B. Qiu,J. Zhang et al., “Intensity-modulated radio- therapy versus three-dimensional conformal radiotherapy in definitive chemoradiotherapy for cervical esophageal squa- mous cell carcinoma: comparison of survival outcomes and toxicities,” Cancer Research and Treatment, vol. 52, no. 1, pp. 31–40, 2020.
[26] K. Nam, J. R. Eisenbrey, M. Stanczak et al., “Monitoring neo- adjuvant chemotherapy for breast cancer by using three- dimensional subharmonic aided pressure estimation and imaging with US contrast agents: preliminary experience,” Radiology, vol. 285, no. 1, pp. 53–62, 2017.
[27] M. Jirkovska, T. Novak, B. Malinova, and R. Lohynska, “Three-dimensional conformal radiotherapy versus intensity modulated radiotherapy with simultaneous integrated boost in the treatment of locally advanced head and neck carci- noma,” Neoplasma, vol. 66, no. 5, pp. 830–838, 2019.
[28] J. P. Hoogendam,A. Zaal, E. G. Rutten et al., “Detection of cer- vical cancer recurrence during follow-up: amultivariable com- parison of 9 frequently investigated serum biomarkers,” Gynecologic Oncology, vol. 131, no. 3, pp. 655–660, 2013.
[29] J. Fu, W. Wang, Y. Wang, C. Liu, and P. Wang, “The role of squamous cell carcinoma antigen (SCCag) in outcome predic- tion after concurrent chemoradiotherapy and treatment deci- sions for patients with cervical cancer,” Radiation Oncology, vol. 14, no. 1, p. 146, 2019.
[30] N. Kim, W. Park, W. K. Cho et al., “Significance of serum CA125 level in surgically resected cervical adenocarcinoma with adverse features,” Journal of Gynecologic Oncology, vol. 32, no. 5, article e72, 2021.
[31] L. Zhang,Q. Chen,J. Hu, Y. Chen, C. Liu, and C. Xu, “Expres- sion of HIF-2alpha and VEGF in cervical squamous cell carcinoma and its clinical significance,” BioMed Research International, vol. 2016, Article ID 5631935, 7 pages, 2016.
[32] L. Li,X. Song,R. Liu et al., “Chemotherapy versus radiotherapy for FIGO stages IB1 and IIA1 cervical carcinoma patients with postoperative isolated deep stromal invasion: a retrospective study,” BMC Cancer, vol. 16, no. 1, p. 403, 2016.
[33] Z. Shi, J. Vasquez-Vivar, K. Luo et al., “Ascending lipopolysaccharide-induced intrauterine inflammation in near-term rabbits leading to newborn neurobehavioral defi - cits,” Developmental Neuroscience, vol. 40, no. 5-6, pp. 534– 546, 2019.
[34] L. Deng, X. Li, Z. Shi, P. Jiang, D. Chen, and L. Ma, “Maternal and perinatal outcome in cases offulminant viral hepatitis in late pregnancy,” International Journal of Gynecology & Obstet- rics, vol. 119, no. 2, pp. 145–148, 2012.
[35] Z. Shi, K. Luo, S. Janiet al., “Mimicking partial to total placen- tal insufficiencyin a rabbit model of cerebral palsy,” Journal of Neuroscience Research, 2021.
[36] Y. Yang, L. Deng, X. Li et al., “Evaluation of the prognosis of fulminant viral hepatitis in late pregnancy by the MELD scor- ing system,” European Journal of Clinical Microbiology & Infectious Diseases, vol. 31, no. 10, pp. 2673–2678, 2012.
[37] Y. Yang, L. Deng,X. Li etal., “Analysis of prognosis-associated factors in fulminant viral hepatitis during pregnancy in China,” International Journal of Gynaecology and Obstetrics, vol. 114, no. 3, pp. 242–245, 2011.
[38] X. M. Li, L. Ma, Y. B. Yang, Z. J. Shi, and S. S. Zhou, “Prognos- tic factors offulminant hepatitis in pregnancy,” Chinese Med- ical Journal, vol. 118, no. 20, pp. 1754–1757, 2005.
[39] Z. Shi, L. Ma, H. Wanget al., “Insulin and hypertonic glucose in the management of aseptic fat liquefaction of post-surgical incision: a meta-analysis and systematic review,” International Wound Journal, vol. 10, no. 1, pp. 91–97, 2013.
初审:张莉红 复审:张 洁
