Upregulation of miR‑519 enhances radiosensitivity of esophageal squamous cell carcinoma trough...
Upregulation of miR‑519 enhances radiosensitivity of esophageal squamous cell carcinoma trough targeting PI3K/AKT/mTOR signaling pathway
Cancer Chemotherapy and Pharmacology
Yanshan Zhang1 · Weizuo Chen1 · Huijuan Wang2 iD · Tingting Pan1 · Yinguo Zhang3 · Chao Li3
© Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract
Purpose MicroRNA-519 (miR-519) has been previously reported to function as a tumor suppressor in several types of malignancies. This study aimed to probe the biological role of miR-519 in esophageal squamous cell carcinoma (ESCC).
Methods qRT-PCR was utilized to test the miR-519 expression level in ESCC tissues and cells. Clinical value of miR-519 was investigated by Kaplan–Meier method. Function assays were conducted to determine the role of miR-519 in radioresistance of ESCC cells. The miR-519-regulated pathways were determined by Kyoto Encyclopedia of Genes and Genomes pathway analysis.
Results Low expression level of miR-519 was closely correlated with the poor prognosis for overall survival of ESCC patients or patients who received radiotherapy. Functional assays indicated that upregulation of miR-519 made ESCC cells more sensitive to γ-ray radiation and facilitated ESCC cell apoptosis triggered by irradiation treatment via regulating DNA response. Ectopic expression of miR-519 decreased the level of p-AKT and p-mTOR, thus inactivating PI3K/AKT/mTOR signaling pathway after irradiation.
Conclusion These observations elucidated that upregulated miR-519 is closely correlated with the radiosensitivity of ESCC cells, which may contribute to finding a new promising target for improving the efficiency of radiotherapy in patients with ESCC.
Keywords miR-519 · ESCC · Radiosensitivity · PI3K/AKT/mTOR
Introduction
Esophageal carcinoma (EC) is a highly malignant human cancer with approximately 509,000 deaths cases per year [1]. Esophageal squamous cell carcinoma (ESCC), the most frequent type of EC. Recent years, radiotherapy has been widely applied to the treatment for ESCC patients, thus becoming a critical part in therapeutic strategies [2,3]. Disappointingly, approximately 40–60% of patients maintain refractory or eventually relapse due to resistance to radiotherapy [4, 5]. Thus, studies on the sensitivity to radiotherapy are promising for promoting the therapeutic efficacy of cancers.
MicroRNAs (miRNAs) represent a class of small, noncoding RNAs with 19–23 nucleotides that negatively modulate gene expression through interacting with various mRNAs so as to inhibit mRNA translation or lead to mRNA degradation [6]. In recent researches, miRNAs have been illustrated to play vital roles in biological or pathological processes including tumor initiation and progression [7–12]. What’s more, increasing miRNAs have been verified to play a role in regulating radioresistance of cancers including ESCC. For example, microRNA-381 enhances radiosensitivity in esophageal squamous cell carcinoma by targeting X-linked inhibitor of apoptosis protein [13], miRNA-200c enhances radiosensitivity of esophageal cancer by targeting p21 [14]. miR-519 is a tumor suppressor in multiple cancers, such as nasopharyngeal carcinoma, colorectal cancer and cervical cancer [15–19]. However, its role in ESCC remains poorly understood.
In this study, we mainly aimed to investigate the effect of miR-519 on the radiosensitivity of ESCC.
Materials and methods
Tissue specimens
Ninety fresh ESCC tissue samples and paired histologically normal tissues were obtained from patients undergone surgery at Tumor Hospital of Wuwei. After separated by experienced pathologists, all 90 pairs of ESCC tissues and adjacent noncancerous tissues were frozen immediately using liquid nitrogen and then stored at −80 °C until use. All of the patients received no treatments, such as chemotherapy, radiotherapy and adjuvant therapy before surgery and signed informed consent for the use of tumors excised. After surgery, 47 of 90 patients underwent concurrent chemotherapy with radiotherapy, while the other 43 received chemotherapy only. The use of samples was approved by the Ethics Committee of Tumor Hospital of Wuwei.
RNA extraction and quantitative reverse transcription‑polymerase chain reaction (qRT‑PCR)
Total RNA was extracted from 90 ESCC tissues and paired normal tissues using the Absolutely RNA™ Miniprep kit (Stratagene, Santa Clara, CA) in line with the manufacturer’s protocols, and RNA quantification was completed by a DUVR 800 UV/Vis Spectrophotometer (Beckman Coulter, Fullerton, CA). Subsequently, the cDNAs were obtained using TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The real-time PCR was performed using SYBR Green in an ABI 7500 real-time PCR system. U6 snRNA was used as the normalized control. The primers for miR-519 were provided by ABI PRISM (ABI PRISM, Carisbad, CA). Relative expression of miR-519 was calculated using 2−ΔΔCt method.
Cell culture
Human esophageal epithelial cell line Het-1A were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China), while the ESCC cell lines (KYSE450, EC109, EC9706 and KYSE150) were purchased from the tumor cell bank of the Chinese Academy of Medical Science. All cells were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100U/ml penicillin, and 100 μg/ml streptomycin (Hyclone, Logan, UT, USA) at 37 °C with atmosphere of 5% CO2.
Plasmid construction and cell transfection
The sequence of pre-miR-519 was sub-cloned into the pSuppressorNeo expression vector to construct miR-519 mimics, and the empty vector was the negative control (miR-NC). Then the KYSE150 and KYSE450 cells were transfected with miR-519 mimics or miR-NC using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in the light of the manufacturer’s instructions.
Ionizing radiation
KYSE150 or KYSE450 cells were exposed to irradiation treatment at different doses in a JL Shepherd Model 143 and 137Cesium γ-irradiated at a speed of 2.4 Gy/min.
Colony formation assay
A total of 800 cells were seeded into a 6-well plate in triplicate. After cultured overnight, cells were exposed to different doses of γ-rays. Two weeks later, the colonies were fixed using methanol, stained by Giemsa staining and then counted manually.
MTT assays
MTT assay (Sigma-Aldrich) was performed to measure cell proliferation. Briefly, cells (at a density of 5×103 cells/well) were planted in 96-well plates and incubated overnight. Then 20 μl of MTT solution (5 mg/mol) was added, and cells in each well were incubated for 4 h at 37 °C until the addition of 150 μl dimethyl sulfoxide (DMSO). Optical density at a wavelength of 490 nm was measured using a multidetection microplate reader (BMG LABTECH, Cary, NC, USA).
γ‑H2AX foci formation assay
In brief, cells were maintained in cover slips preserved in 35 mm petri plates and treated with irradiation at 4 Gy and measured in 4 different time points (0, 1, 2 and 4 h). After washed with PBS, cells were fixed in 4% paraformaldehyde at room temperature and then washed twice using PBS before staining. For immunofluorescence staining, cells were permeabilized for 3 min in 0.25% Triton X-100 in PBS, washed twice in PBS and blocked using 5% BSA for 1 h. Thereafter, cells were incubated with primary antibody at room temperature for 1 h followed by incubation with secondary antibody at room temperature for another 1 h. Finally, the rinsed cells were mounted using ProLong Gold antifade with DAPI mounting media (Molecular Probe, USA) and the images were captured with a Carl Zeiss confocal microscope. The primary antibody was rabbit anti-γH2AX (Cell Signaling, USA) and the secondary antibody was Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probe, USA).
Flow cytometry analysis
After 8 h of irradiation with a dose of 0 and 4 Gy, cells were collected to detect apoptosis by the use of the Annexin V-FITC apoptosis detection kit (Sigma). Subsequently, cells were analyzed using flow cytometry (FACScan, BD Biosciences).
Western blot analysis
The proteins extracted from ESCC tissues or cells were separated by SDS-PAGE and subsequently transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked and then incubated with primary antibodies, such as anti-PI3K (sc-376412, Santa Cruz Biotechnology, CA, USA), anti-p-PI3K (ab154598, Abcam, Cambridge, UK), anti-AKT (#4685), anti-p-AKT (#4058), anti-mTOR (#2972), anti-p-mTOR (#2974) (Cell Signaling Technology, Inc., Beverly, MA, USA) and GAPDH (sc-293335, Santa Cruz Biotechnology). Afterwards, the membranes were probed with horseradish peroxidase-conjugated secondary antibodies and then visualized with super ECL detection reagent (Applygen, Beijing, China).
Statistical analysis
All statistical analyses were carried out using SPSS 16.0 software. All data from three independent experiments were analyzed using Student’s t test for two groups or one-wayANOVA for more than two groups. Survival curves were determined using the Kaplan–Meier method. P<0.05 was thought to be statistically significant.
Results
The expression of miR‑519 in ESCC tissues
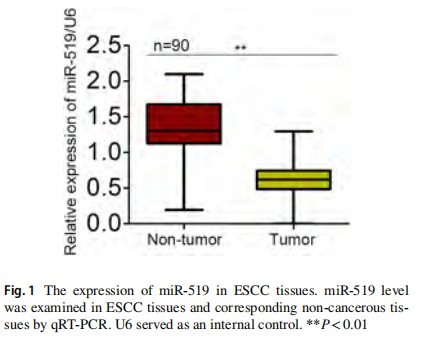
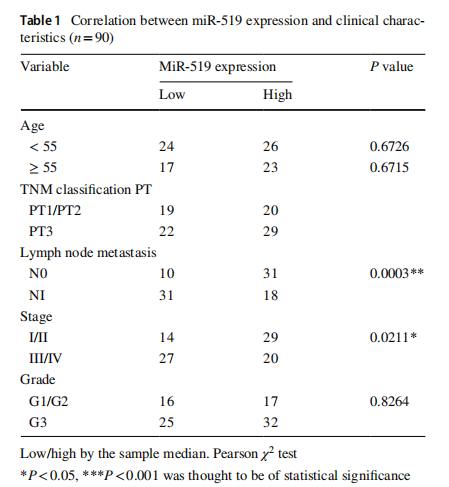
We first examined the expression level of miR-519 in 90 paired ESCC samples and non-tumor samples using qRT-PCR. As shown in Fig. 1, miR-519 expression was markedly lower in ESCC tissues in comparison with the adjacent non-cancerous tissues. Furthermore, we analyzed the association between miR-519 expression and clinicopathological characteristics. Seen from Table 1, we found that the level of miR-519 was associated with lymph node metastasis (P<0.001, Table 1). Furthermore, the Cox regression analysis showed a remarkable prognostic significance of miR-519 expression and TNM stage in ESCC patients (Table 2). These data revealed that downregulated miR-519 may be correlated with the clinical significance for patients with ESCC.
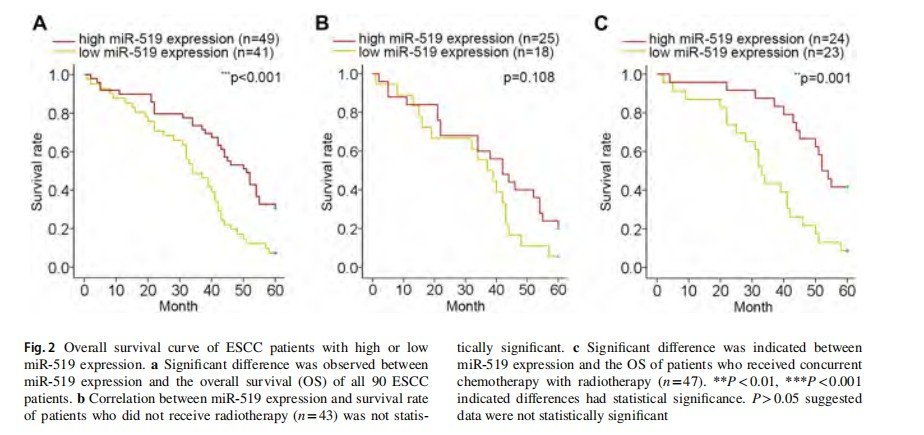
The association between miR‑519 expression and clinical outcomes of ESCC patients
To determine the prognostic potential of miR-519 expression, Kaplan–Meier survival analysis and a log-rank test were performed. Significant relation between miR-519 expression and overall survival (OS) was analyzed and identified (P<0.001, Fig. 2a). In the patients without radiotherapy (43 out of 90), no difference of OS was suggested in ESCC patients with miR-519 high-expression or low expression (P=0.108, Fig. 2b). In contrast, miR-519 expression was positively correlated with the survival rate of patients with concurrent chemotherapy and radiotherapy (47 out of 90) (P=0.001, Fig. 2c). These results suggested that miR-519 may have a predictive value for radiotherapy.
Effects of miR‑519 expression on the radiosensitivity of ESCC cells
Next, we detected miR-519 expression in Het-1A cells and four ESCC cell lines using qRT-PCR. As displayed in Fig. 3a, miR-519 expression was notably lower in four ESCC cell lines in comparison with Het-1A cell line, and KYSE450 and KYSE150 cells were further used for the following study due to their lower miR-519 expression and high radioresistance identifed as before [13]. Before functional assays, miR-519 was upregulated in KYSE450 and KYSE450 cell lines (Fig. 3b). Cell viability was assessed frst in miR-519-upregulated cells. Results of MTT assay revealed that the viability of two ESCC cells was attenuated by the upregulation of miR-519 (Fig. 3c). Afterwards, colony formation assay was conducted to evaluate the impact of miR-519 expression on the radiosensitivity of ESCC cells. The survival fraction of KYSE450 and KYSE150 cells under miR-519 overexpression was noticeably lower than that of control groups (Fig. 3d, e), indicating a positive correlation between miR-519 expression and sensitivity of ESCC cells to gamma radiation. Flow cytometry analysis elucidated that apoptosis was induced by miR-519 overexpression (Fig. 3f). Apoptosis was promoted more efficiently by the upregulation of miR-519 when ESCC cells were treated with 4 Gy irradiation. Furthermore, apoptosis-related proteins were detected in miR-519-upregulated ESCC cells treated with or without irradiation. The increased protein levels of Bax and Cleaved-caspase 3 were tested in cells transfected with miR-519 mimics after irradiation (Supplementary Fig. 1A, B). However, the protein level of Bcl-2 was decreased after upregulation of miR-519 and treatment with irradiation. Furtherly, migration was detected in cells transfected with miR-519 mimics after treatment with or without irradiation. As a result, increased level of miR-519 impaired cell migration. The inhibitory effect of miR-519 on cell migration was more efficient after treatment with irradiation (Supplementary Fig. 1C). These results indicated that upregulation of miR-519 could elevate radiosensitivity of ESCC cells.
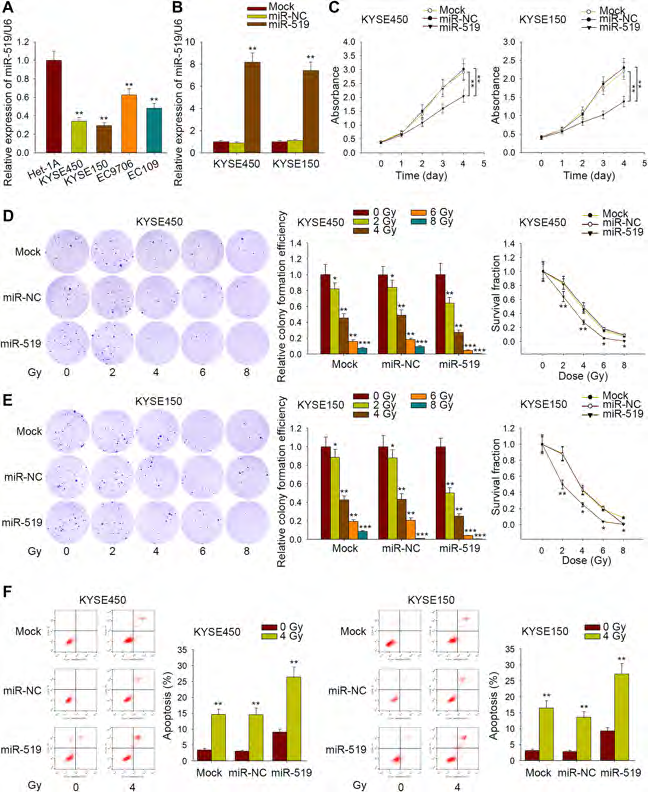
Fig. 3 Impacts of miR-519 overexpression on the radiosensitivity of ESCC cells. a qRT-PCR results of miR-519 expression in four ESCC cell lines and normal Het-1A cells. b The level of miR-519 was sharply increased after positive transfection of miR-519 in KYSE450 and KYSE150 cells. c MTT assay was carried out to determine the effect of miR-519 on ESCC cell viability. d Colony formation assay was conducted to assess the radiosensitivity of KYSE450 and KYSE150 cells with no transfection (Mock), or cells transfected with miR-NC or miR-519. e Apoptosis rate of miR-519-upregulated KYSE150 and KYSE450 cells were measured after treatment with or without irradiation. *P<0.05, **P<0.01, ***P<0.001 indicated difference had statistical significance
miR‑519 enhanced radiosensitivity by regulating the repair of DNA double-strand breaks and PI3K/AKT/mTOR signaling
Treatment with irradiation can induce DNA repair in cancer cells. Here, we further identify whether expression influenced the repair ability of DNA damage caused by irradiation, the γ-H2AX foci formation assays were conducted after irradiation at four different time points (0 h, 1 h, 2 h, 4 h). As demonstrated in Fig. 4a, the number of γ-H2AX foci in KYSE450 and KYSE150 cells upon miR-519 upregulation was pronouncedly higher than that in control cells after 4 Gy irradiation. To analyze the downstream signaling pathway regulated by miR-519, we searched out the potential downstream mRNAs of miR-519 from targetScan. These mRNAs were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway. It was found that PI3K/AKT signaling pathway was potentially regulated by miR-519 (Fig. 4b). The western blot results showed that miR-519 overexpression in both KYSE450 and KYSE150 cells led to a decrease of p-PI3K, p-AKT and p-mTOR level, while irradiation further aggravated this effect (Fig. 4c). These data show that miR-519 expression may function in ESCC through inhibiting PI3K/AKT/mTOR pathway.
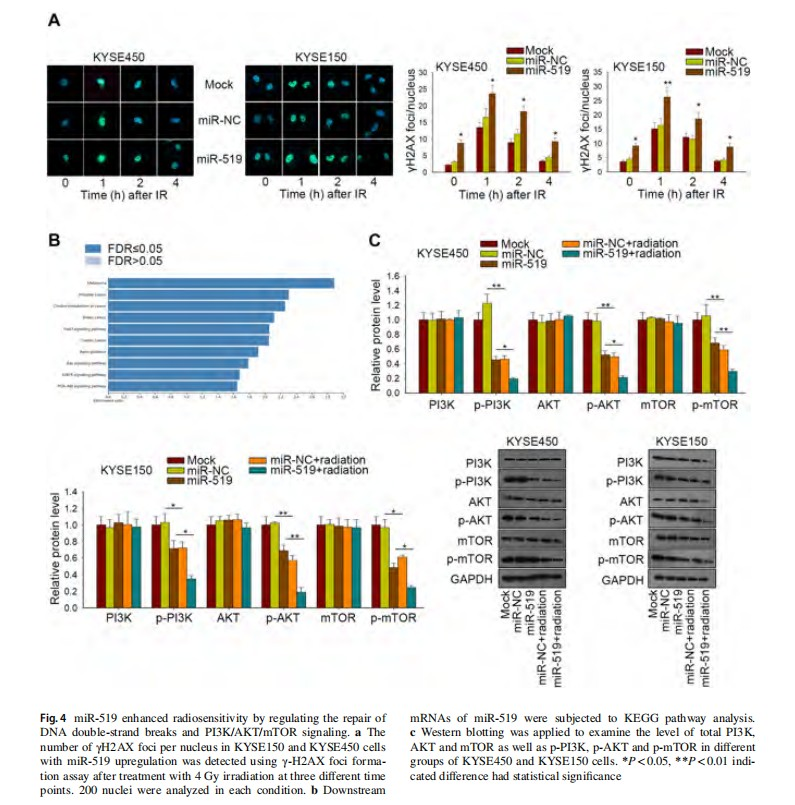
PI3K/AKT/mTOR signaling pathway involved in miR‑519-mediated radiosensitivity
To make sure whether miR-519 affect the radiosensitivity of ESCC cells via this pathway, the PI3K activator, 740Y-P was applied to carry out the rescue assays. First, we demonstrated that the addition of 740Y-P increased the decreased phosphorylation level of PI3K, AKT and mTOR, which were induced by miR-519 with or without radiation in KYSE150 cells (Fig. 5a). Seen from Fig. 5b, c, miR-519 upregulation led to decreased cell proliferative ability while together with irradiation treatment resulted in further impaired proliferative ability, both of which could be partially enhanced by the co-treatment of 740Y-P in KYSE150 cells. In contrast, the cell apoptosis rate of KYSE150 cells facilitated by miR-519 overexpression was declined by the use of 740Y-P, while the further increased apoptosis induced by miR-519 overexpression and irradiation was also partially attenuated by 740Y-P (Fig. 5d). Collectively, the activation of PI3K/AKT/mTOR pathway restored miR-519 overexpression promoted radiosensitivity of ESCC cells. In other words, miR-519 upregulation enhanced radiosensitivity of ESCC via inactivating PI3K/AKT/mTOR signaling pathway.
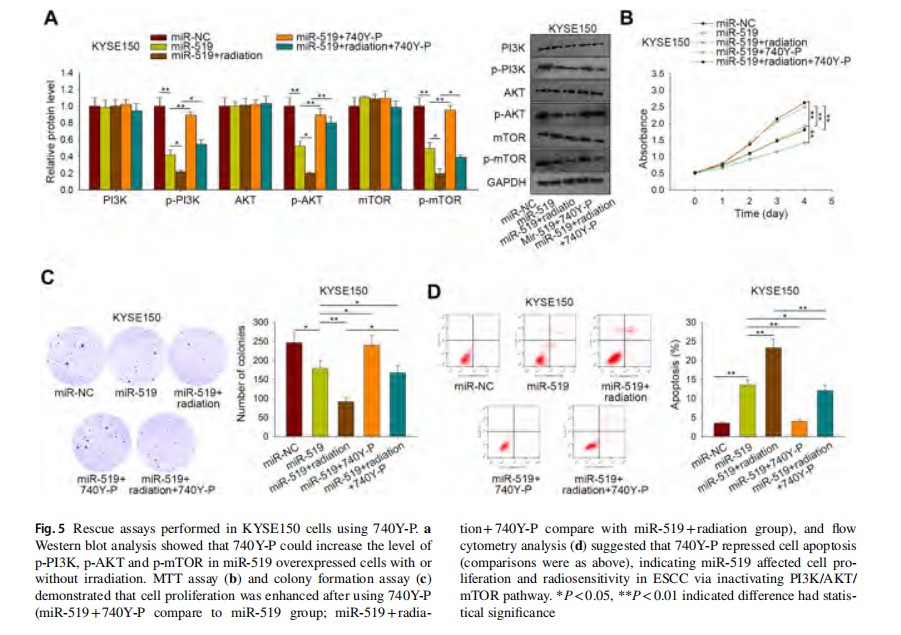
Discussion
Researches have revealed that the abnormal expression of miRNAs are strongly correlated with the clinicopathologi-cal features of malignant tumors, such as histological type, differentiation, stage, metastasis, response to therapy and prognosis [20]. Hence, it is of great importance of miRNAs in cancer diagnosis, treatment and prognosis. In the past decades, miRNAs have been reported to play key roles in various cancers [21, 22]. Besides, there are also many miRNAs have been identifed to be functional in ESCC [23, 24]. What’s more, miRNAs can regulate the responses to therapeutics in cancers [20, 25–29]. Furthermore, miRNAs are also found to be participated in the regulation of ESCC radiosensitiv-ity. For example, increased miRNA-22 expression sensitizesESCC to irradiation [30]; MiR-338-5p enhances the radiosensitivity of ESCC through targeting surviving to induce apoptosis [31]. miR-519, a tumor suppressor, which has been identifed in several carcinomas, including nasopharyngeal carcinoma, colorectal cancer, cervical cancer [15–19].
In the present study, we found that miR-519 was expressed at a low level in ESCC tissues compared with the corresponding non-cancerous tissues, which was in accord with those previous researches [15–19]. Meanwhile, miR-519 level predicted poorer overall survival in all 90 ESCC patients as well as those underwent radiotherapy. Based on this, we suspected that miR-519 expression might be associ-ated with radiosensitivity of ESCC patients. This suspicion was validated after a series of gain of function experiments.
Next, we explored the downstream molecular mecha-nism of miR-519, which help miR-519 exert functions inradiosensitivity of ESCC cells. Previous studies have shown that dysregulation of genes in radioresistance can induce the DNA damage checkpoint response and increase the capac-ity for DNA repair [32, 33]. PI3K/AKT, a signal transduction pathway, was closely correlated with cell viability. Abnormal activation of the PI3K/AKT signaling pathway induced abnormal cell growth and differentiation [34]. Phosphorylation of AKT generated p-AKT, increased the expression level of p-mTOR and p-70S6 K through further activating the mTOR pathway, and ultimately increased cell viability [35]. Furthermore, people have indicated the relationship between responses to radiotherapy and PI3K/AKT signaling pathway. For example, BMI-1 suppression increases the radiosensitivity of esophageal carcinoma via the PI3K/AKT signaling pathway [36]; PI3K/AKT/mTOR pathway inhibitors enhance radiosensitivity of radioresist-ant prostate cancer cells [37]. And the full inactivation of p-AKT could be the key to enhancing the radiosensitivity of tumor cells [36]. In this study, overexpression of miR-519 downregulated the level of p-PI3K, p-AKT and p-mTOR. Furthermore, the application of 740Y-P (the PI3K agonist) reversed the cell proliferative ability and apoptosis caused by miR-519 upregulation in ESCC cells treated with irradiation. In conclusion, our study was the first to uncover the promoting role of miR-519 in radiosensitivity of ESCC, thus providing a novel potential biomarker for the radiotherapy of ESCC patients.
Acknowledgements Thanks to all the participators.
Compliance with ethical standards
Conflict of interest Authors declare that they have no confict of inter-est.
Ethical approval This article does not contain any studies with ani-mals performed by any of the authors. The use of these samples from patients was approved by the Ethics Committee of Tumor Hospital of Wuwei.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
2. Berger B, Belka C (2009) Evidence-based radiation oncology: oesophagus. Radiother Oncol 92(2):276-290.https://doi.org/10.1016/j.radonc.2009.02.019
3. Nakajima M, Kato H (2013) Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother 14(10):1345–1354
4. Borghesi S, Hawkins MA, Tait D (2008) Oesophagectomy after definitive chemoradiation in patients with locally advanced oesophageal cancer. Clin Oncol (R Coll Radiol) 20(3):221–226. https://doi.org/10.1016/j.clon.2007.12.001
5. Minsky B, Pajak T, Ginsberg R, Pisansky T, Martenson J, Komaki R, Okawara G, Rosenthal S, Kelsen D (2002) INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 20(5):1167–1174
6. Bartel D (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
7. Papagiannakopoulos T, Kosik KS (2008) MicroRNAs: regulators of oncogenesis and stemness. BMC Med 6:15. https://doi.org/10.1186/1741-7015-6-15
8. Palanichamy JK, Rao DS (2014) miRNA dysregulation in cancer: towards a mechanistic understanding. Front Genet 5:54. https://doi.org/10.3389/fgene.2014.00054
9. Dong Y, Zheng Y, Wang C, Ding X, Du Y, Liu L, Zhang W, Zhang W, Zhong Y, Wu Y, Song X (2018) MiR-876-5p modulates head and neck squamous cell carcinoma metastasis and invasion by targeting vimentin. Cancer Cell Int 18:121
10. Song L, Dai Z, Zhang S, Zhang H, Liu C, Ma X, Liu D, Zan Y, Yin X (2018) MicroRNA-1179 suppresses cell growth and invasion by targeting sperm-associated antigen 5-mediated Akt signaling in human non-small cell lung cancer. Biochem Biophys Res Commun 504(1):164–170
11. Xu J, Wang F, Wang X, He Z, Zhu X (2018) miRNA-543 promotes cell migration and invasion by targeting SPOP in gastric cancer. Onco Targets Ther 11:5075–5082
12. Wang L, Yu P, Li B, Guo Y, Liang Z, Zheng L, Yang J, Xu H, Liu S, Zheng L, Zhou H, Qu L (2018) miR-372 and miR-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol Oncol 12(11):1949–1964
13. Zhou S, Ye W, Ren J, Shao Q, Qi Y, Liang J, Zhang M (2015) MicroRNA-381 increases radiosensitivity in esophageal squamous cell carcinoma. Am J Cancer Res 5(1):267–277
14. Zheng R, Liu Y, Zhang X, Zhao P, Deng Q (2017) miRNA-200c enhances radiosensitivity of esophageal cancer by cell cycle arrest and targeting P21. Biomed Pharmacother 90:517–523. https://doi.org/10.1016/j.biopha.2017.04.006
15. Yu G, Zhang T, Jing Y, Bao Q, Tang Q, Zhang Y (2017) miR-519 suppresses nasopharyngeal carcinoma cell proliferation by targeting oncogene URG4/URGCP. Life Sci 175:47–51. https://doi.org/10.1016/j.lfs.2017.03.010
16. Deng W, Wang J, Zhang J, Cai J, Bai Z, Zhang Z (2016) Orai1, a direct target of microRNA-519, promotes progression of colorectal cancer via Akt/GSK3beta signaling pathway. Dig Dis Sci 61(6):1553–1560. https://doi.org/10.1007/s10620-015-4029-6
17. Abdelmohsen K, Srikantan S, Kuwano Y, Gorospe M (2008) miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc Natl Acad Sci USA 105(51):20297–20302
18. Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, Cabo R, Gorospe M (2010) miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle 9(7):1354–1359. https://doi.org/10.4161/cc.9.7.11164
19. Abdelmohsen K, Srikantan S, Tominaga K, Kang MJ, Yaniv Y, Martindale JL, Yang X, Park SS, Becker KG, Subramanian M, Maudsley S, Lal A, Gorospe M (2012) Growth inhibition by miR-519 via multiple p21-inducing pathways. Mol Cell Biol 32(13):2530–2548. https://doi.org/10.1128/MCB.00510-12
20. Cheng G (2015) Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev 81:75–93. https://doi.org/10.1016/j.addr.2014.09.001
21. He H, Hao SJ, Yao L, Yang F, Di Y, Li J, Jiang YJ, Jin C, Fu DL (2014) MicroRNA-218 inhibits cell invasion and migration of pancreatic cancer via regulating ROBO1. Cancer Biol Ther 15(10):1333–1339. https://doi.org/10.4161/cbt.29706
22. Xu W, Hang M, Yuan C, Wu F, Chen S, Xue K (2015) MicroRNA-139-5p inhibits cell proliferation and invasion by targeting insulin-like growth factor 1 receptor in human non-small cell lung cancer. Int J Clin Exp Pathol 8(4):3864–3870
23. Li S-P, Su H-X, Zhao D, Guan Q-L (2016) Plasma miRNA-506 as a prognostic biomarker for esophageal squamous cell carcinoma. Med Sci Monit 22:2195–2201. https://doi.org/10.12659/msm.899377
24. Yu T, Cao R, Li S, Fu M, Ren L, Chen W, Zhu H, Zhan Q, Shi R (2015) MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells. BMC Cancer 15:29. https://doi.org/10.1186/s12885-015-1031-5
25. Chaudhry MA (2014) Radiation-induced microRNA: discovery, functional analysis, and cancer radiotherapy. J Cell Biochem 115(3):436–449. https://doi.org/10.1002/jcb.24694
26. Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20(8):460–469. https://doi.org/10.1016/j.molmed.2014.06.005
27. Metheetrairut C, Slack FJ (2013) MicroRNAs in the ionizing radiation response and in radiotherapy. Curr Opin Genet Dev 23(1):12–19. https://doi.org/10.1016/j.gde.2013.01.002
28. Yu X, Li Z, Yu J, Chan MT, Wu WK (2015) MicroRNAs predict and modulate responses to chemotherapy in colorectal cancer. Cell Prolif 48(5):503–510. https://doi.org/10.1111/cpr.12202
29. Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, Cui SZ, Wang LT (2014) MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol 20(32):11347–11355. https://doi.org/10.3748/wjg.v20.i32.11347
30. Wang XC, Zhang ZB, Wang YY, Wu HY, Li DG, Meng AM, Fan FY (2013) Increased miRNA-22 expression sensitizes esophageal squamous cell carcinoma to irradiation. J Radiat Res 54(3):401–408. https://doi.org/10.1093/jrr/rrs113
31. Park M, Yoon HJ, Kang MC, Kwon J, Lee HW (2017) MiR-338-5p enhances the radiosensitivity of esophageal squamous cell carcinoma by inducing apoptosis through targeting survivin. Sci Rep 7(1):10932. https://doi.org/10.1038/s41598-017-10977-9
32. Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, Yamagishi H (2004) Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer 91(8):1543–1550. https://doi.org/10.1038/sj.bjc.6602187
33. Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y (2002) Classification of sensitivity or resistance of cervical cancers to ionizing radiation according to expression profiles of 62 genes selected by cDNA microarray analysis. Neoplasia 4(4):295–303. https://doi.org/10.1038/sj.neo.7900251
34. Liu G, Song Y, Cui L, Wen Z, Lu X (2015) Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int J Clin Exp Pathol 8(2):1402–1410
35. Li H, Hu J, Wu S, Wang L, Cao X, Zhang X, Dai B, Cao M, Shao R, Zhang R, Majidi M, Ji L, Heymach JV, Wang M, Pan S, Minna J, Mehran RJ, Swisher SG, Roth JA, Fang B (2016) Auranofn-mediated inhibition of PI3K/AKT/mTOR axis andanticancer activity in non-small cell lung cancer cells. Oncotarget 7(3):3548–3558. https://doi.org/10.18632/oncotarget.6516
36. Yang XX, Ma M, Sang MX, Zhang XY, Liu ZK, Song H, Zhu SC (2018) BMI-1 suppression increases the radiosensitivity of oesophageal carcinoma via the PI3K/Akt signaling pathway. Oncol Rep 39(2):667–678. https://doi.org/10.3892/or.2017.6136
37. Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y (2014) PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis 5:e1437. https://doi.org/10.1038/cddis.2014.415
