A study of carbon-ion radiotherapy versus X-ray volumetric modulated arc therapy for lung cancer
A Dosimetric Comparative Study of Carbon-ion Radiotherapy v=Versus X-ray Volumetric Modulated Arc Therapy for Stage III Non-small Cell Lung Cancer
LI Xiaojun, ZHANG Yanshan, REN Yimin, ZHANG Yihe, YE Yancheng, MA Tong, LU Jing, PAN Xin, MA Shuping
Heavy Ion Radiotherapy Department, Wuwei Tumor Hospital of Gansu Province, Gansu Wuwei 733000, China.
【Abstract】 Objective: To examine whether two-dimensional carbon-ion radiotherapy (2D-CIRT) is dosimetrically superior to photon beam volume modulated arc therapy (VMAT) in the treatment of stage III non-small cell lung cancer (NSCLC), and provide basis for clinical carbon ion therapy. Methods: To select 13 patients in our center with stage III NSCLC who were calculated 2D-CIRT by Ciplan planning system and VMAT by Eclipse planning system. The optimization constraints of the two designs were basically the same. Target dose distribution and organs at risk (OARs) doses were evaluated by dose-volume histograms (DVH). SPSS 22.0 software was used for data analysis. Results: Both CIRT and VMAT plans had good tumor coverage with no significant differences in D98, D95, and D50 of PTV1 between the two plans. The homogeneity index (HI) between the two plans were similar (CIRT vs VMAT: 0.39 vs 0.38, P > 0.05). There were significant differences in D98, D95, and D50 of PTV2 between the two plans. The HI of PTV2 was significantly superior in the CIRT plan (CIRT vs VMAT: 0.08 vs 0.16, P < 0.05). The V5, V10, V20, V30, V40, and Dmean of the contralateral lung in the CIRT plan were significantly reduced compared with the photon VMAT. The V5 of the ipsilateral lung was slightly lower in the CIRT plan (CIRT vs VMAT: 53.0% vs 64.4%, P < 0.05). There were no differences between the two groups of the V10, V20, V30, V40, and Dmean. The CIRT had a lower spinal cord Dmax (CIRT vs VMAT: 18.61 Gy vs 43.03 Gy, P < 0.0001), esophageal Dmean (CIRT vs VMAT: 16.25 Gy vs 20.38 Gy, P = 0.031) and V50 (CIRT vs VMAT: 4.49% vs 11.43%, P = 0.005), V10 and V30 of bone, and the V50 of the trachea and bronchial tree. Conclusion: As compared with photon VMAT, 2D-CIRT using passive beam scanning technology significantly reduces radiation dose to the OARs in the treatment of stage III NSCLC, suggesting a better protection of the normal tissues.
【Key words】 Carbon ion radiotherapy, non-small cell lung cancer, volume modulated arc therapy, dosimetric comparison, dose-volume parameters
【Chinese Library Classification】 R734.2
【Document Identification Code】 ADOI: 10.3969/j.issn.1672-4992.2023.02.027
Article Number: 1672-4992-(2023)02-0337-06
Modern Oncology 2023, 31(02): 0337-0343
In 2018, the International Agency for Research on Cancer (IARC) released global cancer statistics, reporting approximately 2.09 million new cases of lung cancer and 1.76 million deaths worldwide. Lung cancer is one of the most common cancers, accounting for 11.6% of total cancer cases and 18.4% of global cancer-related deaths, making it the leading cause of cancer mortality globally[1]. In China, lung cancer ranked first in both incidence and mortality rates in 2015[2]. Non-small cell lung cancer (NSCLC) constitutes 80% of all lung cancer cases, with 35% of patients diagnosed at the locally advanced stage (LA-NSCLC). The overall 5-year survival rate for LA-NSCLC is only 15% to 25%[3]. For patients who cannot tolerate surgery or have unresectable LA-NSCLC, the standard treatment is concurrent chemoradiotherapy. The RTOG0617 study demonstrated that high-dose radiotherapy (74 Gy) does not improve survival in stage III NSCLC patients and increases treatment-related mortality. However, these studies used conventional photon intensity-modulated radiotherapy (IMRT). Currently, particle therapies such as proton and heavy-ion radiotherapy enable precise and adequate tumor irradiation while minimizing damage to normal organs. Carbon ions offer physical and biological advantages over conventional X-rays. The carbon ion beam exhibits a Bragg peak and sharp penumbra, allowing high-dose regions to precisely conform to the tumor target[4-5], significantly sparing normal tissues[6]. Carbon ions are high linear energy transfer (LET) radiation, with a biological effectiveness approximately three times that of X-rays[7-8], and a lower oxygen enhancement ratio, enhancing tumor kill and treatment safety[9-11]. Given these advantages, it is essential to conduct dosimetric studies to determine whether high-dose carbon ion radiotherapy yields different clinical outcomes compared to high-dose photon radiotherapy. This study employs a high prescription dose of 72 Gy/Gy [relative biological effectiveness (RBE)] (RBE = 3.0) for stage III NSCLC, comparing the dosimetric differences between two-dimensional carbon ion radiotherapy (2D-CIRT) and photon volumetric modulated arc therapy (VMAT) plans in target coverage and organ-at-risk (OAR) sparing, to evaluate their respective merits and therapeutic value. The report is as follows.
1 Materials and Methods
1.1 General Information
Thirteen patients with stage III NSCLC who received passive carbon ion beam scanning 2D-CIRT at our center between 2018 and 2021 were included. Patients who could not be treated with a single field due to the maximum field size of 12 cm × 12 cm under the multi-leaf collimator of the carbon ion radiotherapy device were excluded. Ten patients had right lung cancer, and three had left lung cancer. Clinical stages included T2-3 N1-2 M0 (n = 8) and T4N2M0 (n = 5) (Table 1). This study complies with the ethical standards set by the Clinical Trial Ethics Committee of Wuwei Tumor Hospital, Gansu Province (Ethics Approval No.: 2021-Ethics Review-36). All patients provided informed consent and had complete clinical data.
 1.2 Instrumentation
1.2 Instrumentation
This study primarily utilized the Philips Brilliance CT Big Bore with a real-time position management (RPM) system for 4D-CT simulation and positioning of patients. The photon radiotherapy planning system employed was the Varian Eclipse 15.5, and the radiotherapy delivery device was the Varian Vital Beam linear accelerator, equipped with 40 pairs of 0.5 cm and 20 pairs of 1.0 cm multi-leaf collimators (MLCs) and a cone-beam CT (CBCT) image registration system. The HIMM carbon ion medical accelerator, developed by the Institute of Modern Physics, Chinese Academy of Sciences in Lanzhou, was used for carbon ion radiotherapy. The treatment planning system ci-Plan, also developed by the Institute of Modern Physics, was specifically designed for high-energy carbon ion beam radiotherapy planning.
1.3 Treatment Planning
For treatment planning, free-breathing CT images with a slice thickness of 2.5 mm were acquired. The gross tumor volume (GTV) included the primary tumor and clinically positive lymph nodes. Clinically positive lymph nodes were defined as those ≥1 cm on CT or positron emission tomography (PET)-CT images. The clinical target volume (CTV) encompassed the GTV and prophylactic lymph node drainage areas, defined as the ipsilateral hilar and mediastinal lymph node regions according to the International Association for the Study of Lung Cancer (IASLC) lymph node map[12]. The planning target volume (PTV1) was defined as the CTV with a 5 mm margin in all directions, accounting for organ motion and setup errors. PTV2 excluded the prophylactic lymph node regions and was defined as the GTV with a 5 mm setup error margin. Organs at risk (OARs), including the lungs, spinal cord, esophagus, bones (ribs, clavicle, and vertebrae), and the "trachea and proximal bronchial tree (trachea and PBT)," were contoured using the RTOG 1106 OAR Atlas[13].
For carbon ion radiotherapy, a two-dimensional (2D) treatment plan was developed. The principle involved using scanning magnets to laterally expand the pencil beam to create the irradiation field, shaping the target volume in the beam direction with MLCs, and adjusting the beam energy with a range shifter to modify the depth of the Bragg peak within the body. A ridge filter was used to broaden the sharp Bragg peak of the monoenergetic beam into a spread-out Bragg peak (SOBP) matching the tumor thickness, and a surface compensator ensured that the distal edge of the SOBP aligned with the deepest edge of the tumor target. For photon radiotherapy, a volumetric modulated arc therapy (VMAT) plan was employed. Both carbon ion and X-ray radiotherapy plans used the same target volumes and OARs, with target delineation following the recommendations of the International Commission on Radiation Units and Measurements (ICRU) Reports 50, 62, and 83.
The dose distribution for carbon ion passive uniform scanning was calculated using ci-Plan. The dose to PTV1 was Gy (RBE), and PTV2 received a prescription dose of 72 Gy (RBE). The dose for carbon ion radiotherapy was expressed as Gy (RBE), defined as the physical dose (Gy) multiplied by the relative biological effectiveness (RBE) of carbon ions. A two-field approach with horizontal (90°) and vertical (0°) beams was used. The dose constraints for OARs in carbon ion radiotherapy were based on the dose limits for systemic OARs in ion therapy[14]: spinal cord Dmax < 30 Gy (RBE), heart and pericardium V40 (RBE) < 40%, V30 (RBE) < 50%, bilateral lung Dmean < 14 Gy (RBE), single lung V20 (RBE) < 20%, V10 (RBE) < 30%, V5 (RBE) < 40%, and esophagus Dmax < 60 GyE.
For the VMAT plan, the X-ray dose distribution was calculated using Eclipse 15.5. The dose to PTV1 was Gy, and PTV2 received a prescription dose of 72 Gy. The dose for photon radiotherapy was expressed as Gy, defined as the physical dose (Gy) multiplied by the RBE of photons (RBE = 1.0). A coplanar dual-arc VMAT technique was used, with gantry rotation angles of 181°-179° clockwise and 179°-181° counterclockwise. The dose constraints for OARs in photon radiotherapy were based on the QUANTEC[15-16] reports: spinal cord Dmax ≤ 45 Gy, heart V30 ≤ 40%, V40 ≤ 30%, bilateral lung V5 ≤ 60%, V20 ≤ 30%, and V30 ≤ 20%. Inverse optimization was performed using these constraints. Figure 1 illustrates the typical dose distributions for carbon ion and X-ray radiotherapy.
The following dose parameters were evaluated: target coverage (D2, D50, D95, D98) and the homogeneity index (HI) of the PTV. The HI was calculated using the formula HI = (D2% - D98) / D50[17], as per ICRU guidelines, where D2 is the dose received by 2% of the PTV volume, D98 is the dose received by 98% of the PTV volume, and D50 is the dose received by 50% of the PTV volume. A HI closer to 0 indicates better dose homogeneity[18]. Additionally, the following were assessed: spinal cord maximum dose (Dmax); esophageal mean dose (Dmean) and V50; bone V10, V30, and V50; and trachea and PBT V40 and V50. Due to minimal dose differences to organs such as the stomach and small intestine, these were not statistically analyzed. The focus was on lung dose, evaluating the mean dose (Dmean) to the contralateral lung and the ipsilateral lung excluding the GTV, as well as low-dose regions (V5 and V10) and high-dose regions (V20, V30, V40, V50). The dose-volume histograms (DVHs) and dose distributions of the two plans were compared, with the maximum dose point located within the GTV and not in normal tissues, ensuring that normal organs did not exceed tolerance doses.
1.4 Statistical Analysis
Statistical analysis was performed using SPSS 22.0 software. Data were described as mean ± standard deviation, and t-tests or non-parametric tests were conducted. A P-value < 0.05 was considered statistically significant.
2 Results
2.1 Comparison of Target Coverage and Dose Distribution
Both the CIRT and VMAT plans for all 13 patients provided adequate coverage of the PTV and met our requirements. Figure 1 shows a typical comparison of the cross-sectional dose distributions between the CIRT and VMAT plans, with 100% of the prescription dose covering 95% of the target volume. The dose distributions within the target volume were similar. The low-dose radiation area in the contralateral lung was significantly reduced in the CIRT plan compared to the VMAT plan (Figure 1A), with only a small low-dose region observed in the CIRT plan due to the "tail" effect behind the Bragg peak (Figure 1B).
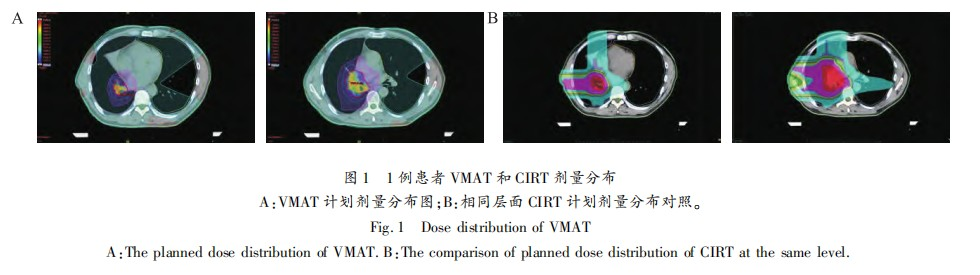
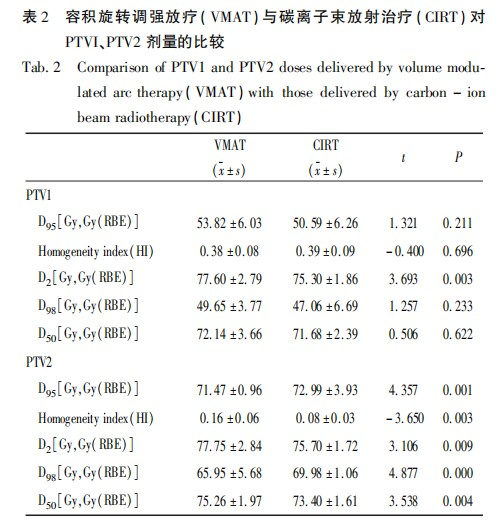
Table 2 shows the DVH for the PTV. There were no statistically significant differences in D95, D98, and D50 for PTV1 between the two treatment plans, and the homogeneity index (HI) for PTV1 also showed no significant difference (CIRT vs VMAT: 0.39 vs 0.38, P = 0.696). Compared to VMAT, the D2 for CIRT was lower [CIRT vs VMAT: 75.30 Gy (RBE) vs 77.60 Gy, t = 3.693, P = 0.003]. According to the ICRU Report 83, due to the large calculation error in point doses, it is recommended to use D2 (Near Maximum) instead of Dmax. This indicates that the dose hotspot in the CIRT plan is lower than that in the photon plan. For PTV2, there were statistically significant differences in D95, D98, D50, and D2 between the two plans. The D95 for PTV2 in the CIRT plan was superior to that in the VMAT plan [CIRT vs VMAT: 72.99 Gy (RBE) vs 71.47 Gy, t = 4.357, P = 0.001]. Additionally, the HI for PTV2 in the CIRT plan was also better than that in the VMAT plan (CIRT vs VMAT: 0.08 vs 0.16, t = -3.650, P = 0.003).
2.2 Comparison of Dose to Organs at Risk
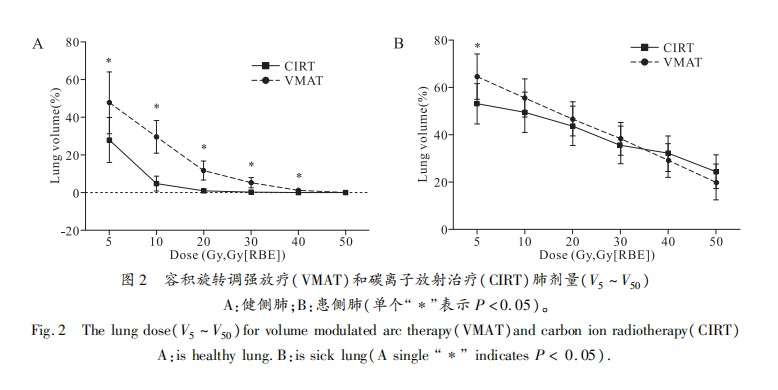
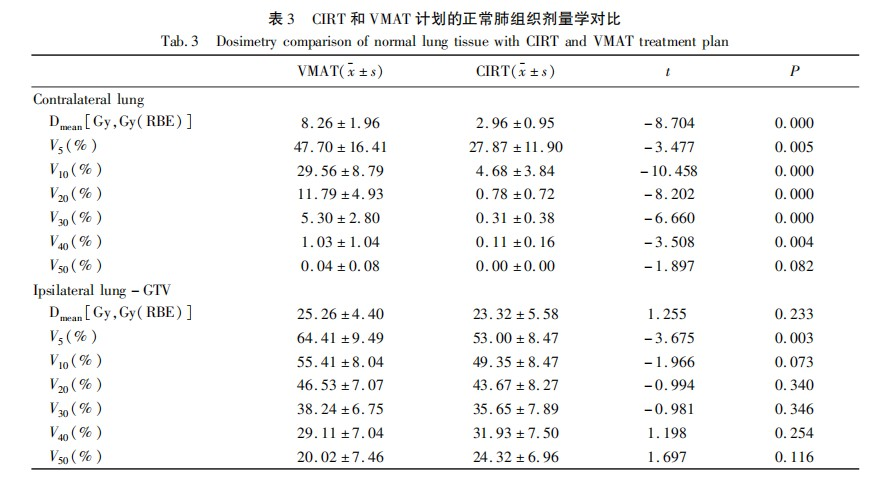
In the contralateral lung, the doses for V5, V10, V20, V30, V40, and Dmean in the CIRT plan were significantly lower than those in the VMAT plan, with statistical differences (Figure 2A, Table 3). The V50 values for both plans were low and showed no statistical difference (CIRT vs VMAT: 0.00 Gy vs 0.04 Gy, P = 0.082). In the ipsilateral lung excluding the GTV, the V5 in the CIRT plan was lower than that in the VMAT plan (CIRT vs VMAT: 53.00 Gy vs 64.41 Gy, t = -3.675, P = 0.003), while there were no significant differences in V10, V20, V30, V40, and Dmean between the two plans (Figure 2B, Table 3).
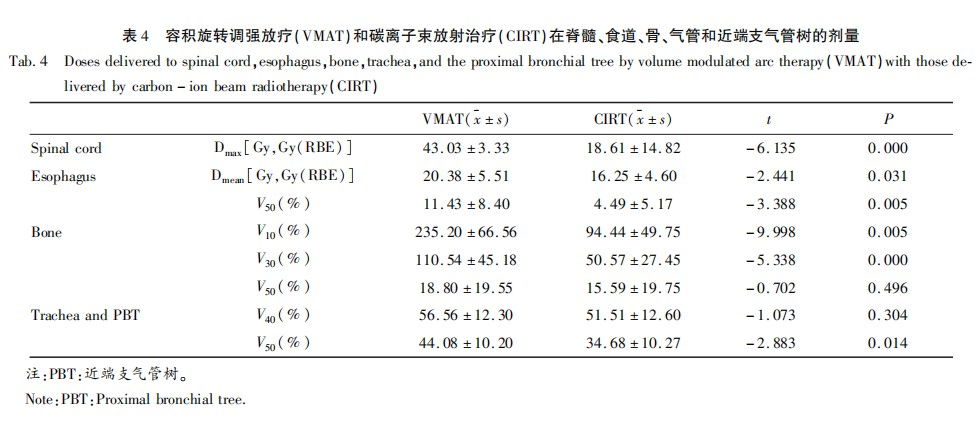
The dose comparisons for other organs at risk are shown in Table 4. The spinal cord Dmax in the CIRT plan was significantly lower than that in the VMAT plan (CIRT vs VMAT: 18.61 Gy vs 43.03 Gy, t = -6.135, P = 0.000). The esophageal Dmean (CIRT vs VMAT: 16.25 Gy vs 20.38 Gy, t = -2.441, P = 0.031) and esophageal V50 (CIRT vs VMAT: 4.49 Gy vs 11.43 Gy, t = -3.388, P = 0.005) in the CIRT plan were lower than those in the VMAT plan, with statistical significance (P < 0.05). The bone V10, V30, and trachea and PBT V50 in the CIRT plan were also lower than those in the VMAT plan (P < 0.05). There were no significant differences in the trachea and PBT V40 and bone V50 between the two radiotherapy methods.
3 Discussion
Lung tumors are surrounded by normal lung tissue, esophagus, heart, spinal cord, ribs, nerves, muscles, and skin. In conventional photon radiotherapy, radiation-induced damage along the irradiation path is a significant concern. A prospective study by the Radiation Therapy Oncology Group (RTOG) found that radiation-induced lung injury, heart disease, esophagitis, and myelitis are the primary dose-limiting factors in radiotherapy for advanced non-small cell lung cancer (NSCLC)[19]. Studies indicate that even with standard treatment combining X-ray radiotherapy and concurrent chemotherapy, the local recurrence rate for stage III NSCLC remains as high as 30%[20-21]. The RTOG0617 study on dose escalation in photon radiotherapy for stage III NSCLC showed that the high-dose group (74 Gy) not only failed to achieve longer median survival compared to the 60 Gy group (20.3 months vs. 28.7 months, P = 0.0042) but also caused harm, with higher incidences of radiation esophagitis and grade III or higher radiation pneumonitis in the high-dose group[22]. COX et al. suggested that the negative effects of the high-dose photon group may be related to cardiopulmonary toxicity[23], and the clinical outcomes of high-dose group patients were worse than those of the standard-dose group. In conventional photon radiotherapy for stage III NSCLC, increasing the prescription dose to the target area inevitably leads to severe complications and reduced survival. The challenge lies in how to increase the prescription dose to the target area while reducing or maintaining the dose to organs at risk (OARs). This brings us to the advantages of carbon ion radiotherapy in particle therapy. Carbon ions possess superior biological and physical properties. Upon entering the tumor, they deposit a large amount of energy in a short time, forming a narrow and sharp Bragg peak with a sharp lateral penumbra, followed by rapid energy attenuation, minimizing scatter to normal tissues behind the tumor. The National Institute of Radiological Sciences (NIRS) in Japan conducted a prospective non-randomized phase I/II clinical trial on carbon ion radiotherapy (CIRT) for locally advanced NSCLC (LA-NSCLC). Patients receiving high-dose 72 GyE carbon ion therapy showed no grade III-V toxicities. The 2-year local control rate (LCR) and overall survival (OS) were 93.1% and 51.9%, respectively, demonstrating significant survival benefits compared to photon radiotherapy alone[24]. Based on this study, we adopted a high prescription dose of 72 Gy (RBE) for stage III NSCLC to explore and elucidate the advantages and therapeutic value of carbon ion radiotherapy from a dosimetric perspective.
This study shows that both the 2D-CIRT and VMAT plans meet the prescription dose requirements. As a two-dimensional plan, the homogeneity index (HI) of PTV1 in the CIRT plan was comparable to that of the photon VMAT plan (Table 2). However, the HI of PTV2 in the CIRT plan was half that of the VMAT plan (0.08 vs. 0.16, P = 0.003), meaning the gap between the maximum and minimum doses in PTV2 was halved, with CIRT providing the lowest dose close to the prescription dose for PTV2. Although VMAT is an advanced radiotherapy technology based on image-guided radiation therapy (IGRT), integrating medical accelerator systems, volumetric imaging systems, imaging systems, dose verification systems, high-precision treatment couch systems, and inverse treatment planning software[25-26], which improves target homogeneity and reduces doses to OARs[27-28], the 2D-CIRT plan can perfectly match the PTV to the Bragg peak region of the carbon ion beam. The dose falloff along the incident path and behind the peak is rapid, and in this study, the dose to PTV2 dropped from 72 to 48, which is more easily achieved with CIRT than with VMAT. This indicates that CIRT can provide uniform dose distribution to tumor tissues even when they are surrounded by normal tissues.
Radiation pneumonitis is one of the main toxicities of chemoradiotherapy for lung cancer, and severe radiation-induced lung injury can be fatal. A narrative review by Yang Yujie et al.[29] in China showed that lung V20 is a critical factor. In patients with lung V20 < 20%, 21%-25%, 26%-30%, and >31%, the incidences of grade 2 or higher radiation pneumonitis were 8.7%, 18.3%, 51%, and 85%, respectively. This demonstrates that even minor differences in V20 can have a significant clinical impact. This study showed that the absolute differences in V20 between CIRT and VMAT for the contralateral and ipsilateral lungs were 11.01% and 2.86%, respectively, while V5 was 19.83% and 11.41% lower in CIRT than in VMAT, indicating that CIRT provides significantly better protection for both lungs than VMAT. According to the study by MARKS et al.[30], the mean lung dose (MLD) is a simple and effective predictor of radiation pneumonitis. Substituting the absolute difference in MLD for the contralateral lung (5.3 Gy (RBE)) from this study into their exponential function formula, the predicted incidence of radiation pneumonitis was 2.94% for CIRT and 5.58% for VMAT, indicating a lower risk of radiation pneumonitis with the carbon ion treatment plan.
The esophagus is anatomically close to the lungs, and radiation esophagitis is an important complication in NSCLC radiotherapy, affecting patients' short-term quality of life and treatment compliance. The mean esophageal dose and V50 can be used to predict the occurrence of radiation esophagitis[31]. In this study, the mean esophageal dose in the CIRT plan was 20% lower than that in the photon VMAT plan, and V50 was 60.7% lower. The doses to the spinal cord, bones (V10, V30), and trachea and proximal bronchial tree (PBT) (V50) in the CIRT plan were also significantly lower than those in the photon VMAT plan. The primary reason is that carbon ions have a sharp Bragg peak, with rapid energy attenuation afterward, minimizing scatter to normal tissues behind the tumor and providing better protection for normal tissues.
In this study, the 2D-CIRT plan for NSCLC did not show advantages in dose distribution for the ipsilateral lung V40, V50, MLD, bone V50, or trachea and PBT V40 compared to the VMAT plan, which is related to passive uniform scanning. During uniform scanning, the beam is longitudinally expanded to the appropriate size by a ridge filter, and the treatment planning system (TPS) calculates the placement of the spread-out Bragg peak (SOBP) accurately on the tumor, achieving passive treatment. It is often required that normal tissues behind the tumor in the beam direction receive little or no irradiation, so tissue compensators are made based on the target distribution to remove the dose behind the tumor by adjusting the compensator. However, some normal tissues along the penetration depth of the carbon ion beam inevitably receive doses comparable to the tumor target. Additionally, the current carbon ion treatment plan at our center is a passive uniform scanning two-dimensional plan with fixed beam paths and limited beam directions, and the TPS lacks inverse optimization capabilities. The modulation ability and calculation accuracy of the target dose need improvement, and the advantages of carbon ions have not been fully utilized. Currently, the optimization of carbon ion spot scanning beam spots has been completed at our center, and the doses to OARs in carbon ion therapy will be further reduced.
In summary, compared to photon volumetric modulated arc therapy (VMAT), the carbon ion two-dimensional radiotherapy (2D-CIRT) plan demonstrates more significant advantages in target dose coverage, homogeneity, and protection of normal tissues, with reduced toxicity. It can be considered a safe, effective, and low-toxicity first-line radiotherapy option for stage III NSCLC.
Reference
[1] FREDDIE, BRAY, JACQUES, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: A Cancer Journal for Clinicians, 2018, 68(6): 394-424.
[2] YANG D, LIU Y, BAI C, et al. Epidemiology of lung cancer and lung cancer screening programs in China and the United States[J]. Cancer Lett, 2020, 468(1): 82-87.
[3] MITHOOWANI H, FEBBRARO M. Non-small-cell lung cancer in 2022: A review for general practitioners in oncology[J]. Curr Oncol, 2022, 29(3): 1828-1839.
[4] BAEK WY, BRAUNROTH T, ROSALES LD LF, et al. Stopping power of water for carbon ions with energies in the bragg peak region[J]. Physical Review E, 2020, 102(6): 81-90.
[5] UENO K, MATSUURA T, HIRAYAMA S, et al. Physical and biological impacts of collimator-scattered protons in spot-scanning proton therapy[J]. Journal of Applied Clinical Medical Physics, 2019, 20(7): 48-57.
[6] WANG L, HU J, LIU X, et al. Intensity-modulated carbon-ion radiation therapy versus intensity-modulated photon-based radiation therapy in locally recurrent nasopharyngeal carcinoma: a dosimetric comparison[J]. Cancer Management and Research, 2019, 11(8): 7767-7777.
[7] CHATZIPAPAS KP, PAPADIMITROULAS P, EMFIETZOGLOU D, et al. Ionizing radiation and complex DNA damage: Quantifying the radiobiological damage using monte carlo simulations[J]. Cancers (Basel), 2020, 12(4): 799-809.
[8] BUGLEWICZ DJ, WALSH KD, HIRAKAWA H, et al. Biological effects of monoenergetic carbon ions and their associated secondary particles[J]. Front Oncol, 2022, 12(2): 17-28.
[9] PARK JM, KIM JI, WU HG. Technological advances in charged-particle therapy[J]. Cancer Res Treat, 2021, 53(3): 635-640.
[10] MOHAMAD O, YAMADA S, DURANTE M. Clinical indications for carbon ion radiotherapy[J]. Clin Oncol, 2018, 30(5): 317-329.
[11] GOETZ G, MITIC M, MITTERMAYR T, et al. Health technology assessment of carbon-ion beam radiotherapy: a systematic review of clinical effectiveness and safety for 54 oncological indications in 12 tumour regions[J]. Anticancer Research, 2019, 39(4): 1635-1650.
[12] GOLDSTRAW P, CHANSKY K, CROWLEY J, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer[J]. Journal of Thoracic Oncology, 2016, 11(1): 39-51.
[13] KONG FM, RITTER T, QUINT DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus[J]. Int J Radiat Oncol Biol Phys, 2011, 81(5): 1442-1457.
[14] ZHANG Q, KONG L, LIU R, et al. Chinese ion therapy guideline (Version 2020)[J]. Precision Radiation Oncology, 2021, 11(3): 1-11.
[15] MARKS LB, BENTZEN SM, DEASY JO, et al. Radiation dose-volume effects in the lung[J]. International Journal of Radiation Oncology, Biology, Physics, 2010, 76(3): 70-76.
[16] KIRKPATRICK JP, KOGEL A, SCHULTHEISS TE. Radiation dose-volume effects in the spinal cord[J]. Radiotherapy & Oncology Journal of the European Society for Therapeutic Radiology & Oncology, 1993, 76(3): 42-S49.
[17] HODAPP N. The ICRU Report83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT)[J]. Strahlenther Onkol, 2012, 188(1): 97-99.
[18] CREEMERS IHP, KUSTERS JMAM, VAN KOLLENBURG PGM, et al. Comparison of dose metrics between automated and manual radiotherapy planning for advanced stage non-small cell lung cancer with volumetric modulated arc therapy[J]. Phys Imaging Radiat Oncol, 2019, 18(9): 92-96.
[19] RAHAM MV, PURDY JA, EMMA B, et al. Clinical dosevolume histogram analysis for penemonitis after 3D treatment for non-small cell lung cancer (NSCLC)[J]. Int J Radiat Oncol Biol Phys, 1999, 45(2): 3232-3239.
[20] CURRAN WJ, PAULUS R, LANGER CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410[J]. J Natl Cancer Inst 2011, 103(19): 1452-1460.
[21] RODRIGUEZ DE DIOS N, NAVARRO-MARTIN A, CIGAR-RAL C, et al. GOECP/SEOR radiotherapy guidelines for non-small-cell lung cancer[J]. Cancer Radiother, 2022, 13(4): 317-318.
[22] BRADLEY PJD, REBECCA PAULUS BS, KOMAKI PR, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study[J]. Lancet Oncology, 2015, 16(2): 187-199.
[23] COX JD. Are the results of RTOG 0617 mysterious[J]. Int J Radiat Oncol Biol Phys, 2012, 82(3): 1042-1044.
[24] TAKAHASHI W, NAKAJIMA M, YAMAMOTO N, et al. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC)[J]. Cancer, 2015, 121(8): 1321-1327.
[25] AKCAY M, ETIZ D, DURUER K, et al. Dosimetric comparison of single-arc/partial-arc volumetric modulated arc therapy and intensity-modulated radiotherapy for peripheral and central lung cancer[J]. J Cancer Res Ther, 2021, 17(1): 80-87.
[26] CHOW J, JIANG R, KICIAK A, et al. Dosimetric comparison between the prostate intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) plans using the planning target volume (PTV) dose-volume factor[J]. Journal of Radiotherapy in Practice, 2016, 15(03): 263-268.
[27] YU CX, TANG G. Intensity-modulated arc therapy: principles, technologies and clinical implementation[J]. Phys Med Biol, 2011, 56(5): 31-54.
[28] ZHANG W, LI GP, XIN HY. New technique in tumor radiotherapy - volumetric intensity modulated arc therapy[J]. Clinical Engineering, 2011, 26(12): 104-105.
[29] YAN Y, FU J, KOWALCHUK RO, et al. Exploration of radiation-induced lung injury, from mechanism to treatment: a narrative review[J]. Transl Lung Cancer Res, 2022, 11(2): 307-322.
[30] MARKS LB, BENTZEN SM, DEASY JO, et al. Radiation dose-volume effects in the lung[J]. International Journal of Radiation Oncology Biology Physics, 2010, 76(3): 70-76.
[31] YU Y, ZHENG H, LIU L, et al. Predicting severe radiation esophagitis in patients with locally advanced esophageal squamous cell carcinoma receiving definitive chemoradiotherapy: Construction and validation of a model based in the clinical and dosimetric parameters as well as inflammatory indexes[J]. Front Oncol, 2021, 24(11): 82-87.
(Edited and proofread by Wen Qiang)
