Dosimetric Comparison Between Two-Dimensional Heavy Ion Treatment Plans and RapidArc Treatment Plans
Dosimetric Comparison Between Two-Dimensional Heavy Ion Treatment Plans and RapidArc Treatment Plans for Multiple Brain Metastases
DOSIMETRIC COMPARISON OF TWO-DIMENSIONAL HEAVY ION TREATMENT PLAN VERSUS RAPIDARC TREATMENT PLAN IN THE TREATMENT OF MULTIPLE BRAIN METASTASES WANG Ziheng,KOU Hairui,MA Xiaoyun,ZHANG Yanshan,QIN Tianyan,GUO Meiju,MENG Wanbin,MENG Li (Department of Radiation Physics,Gansu Wuwei Tumor Hospital,Wuwei 733000,China)
[ABSTRACT] Objective To compare the dosimetric difference between two-dimensional heavy ion treatment plan and RapidArc treatment plan based on a multi-leaf collimator in the treatment of multiple brain metastases. Methods A total of 12 patients with multiple brain metastases(3-5 brain metastases for each patient)who were admitted to Gansu Wuwei Tumor Hospital from May 2020 to May 2021 and were diagnosed and treated with RapidArc or two-dimensional heavy ion treatment were enrolled,and the two-dimensional heavy ion treatment plan and the RapidArc treatment plan were redesigned for each patient.The two treatment plans were compared in terms of dosimetric parameters. Results There were significant differences between the RapidArc treatment plan and the two-dimensional heavy ion treatment plan in homogeneity index and gradient index(t=6.199,z=-4.100,P<0.05),while there was no significant difference in conformity index between the two treatment plans(P>0.05).In the low-dose region(V₅,V₁₀,and V₁₅),the two-dimensional heavy ion treatment plan had a significantly lower irradiated volume of normal brain tissue than the RapidArc treatment plan(t=4.285,3.441,z=-2.309,P<0.05);in the high-dose region(V₃₀),the two-dimensional heavy ion treatment plan had a significantly higher irradiated volume of normal brain tissue than the RapidArc treatment plan(z=-3.233,P<0.05);there was no significant difference between the two treatment plans in the middle dose region(V₂₀ and V₂₅)(P>0.05).As for the maximum dose of the organs at risk,compared with the RapidArc treatment plan,the two-dimensional heavy ion treatment plan had significantly lower maximum doses of the left and right optic nerves,the optic chiasm,and the left and right lens(z=-4.315~-2.488,P<0.05),while there was no significant difference in the maximum dose of the brainstem between the two treatment plans(P>0.05). Conclusion Under the premise of guaranteeing the dose coverage of target volume,heavy ion treatment has unique dosimetric features and can reduce the irradiated volume of normal brain tissue in the low-dose region(V₅,V₁₀,and V₁₅)and better protect normal brain tissue and the organs at risk.
[KEY WORDS] Heavy ion radiotherapy;Photon radiation therapy;RapidArc;Radiotherapy,intensity-modulated;Radiotherapy dosage;Brain neoplasms;Neoplasm metastasis;Dosimetric comparison.
Approximately 170,000 cancer patients worldwide develop brain metastases annually[1].The incidence of brain metastases in adult cancer patients is about 10–20%[2], and this rate is increasing yearly[3]. Among them, 70–80% of patients present with multiple metastases. With advancements in radiotherapy techniques, patients with multiple inoperable brain metastases can achieve desired clinical outcomes through radiotherapy [5-6], significantly prolonging survival. RapidArc is a photon radiotherapy technique based on a linear accelerator. It utilizes multileaf collimator (MLC) motion combined with gantry rotation, modulating real-time dose rates and fluence at different incident angles to achieve volumetric modulated arc therapy (VMAT). This allows simultaneous treatment of multiple brain metastases using a single isocenter, demonstrating high treatment efficiency and superior dose coverage[7]. Heavy ion radiotherapy is one of the most advanced tumor radiotherapy technologies. Unlike photon radiotherapy, heavy ion beams form a Bragg peak and exhibit higher biological effectiveness. By precisely controlling particle transport in equipment and the human body, energy is deposited within the tumor target volume, resulting in superior physical dose distribution, stronger biological effects, higher local control rates, and reduced side effects. This study focused on patients diagnosed with multiple brain metastases at our hospital. RapidArc treatment plans and heavy ion 2D treatment plans were designed, and dosimetric parameters of both plans were compared.
1. Objects and Methods
1.1 Research Subjects
Twelve patients diagnosed with multiple brain metastases at Gansu Wuwei Cancer Hospital between May 2020 and May 2021 were selected. Among them, 3 patients were treated with heavy ion therapy, and 9 patients were treated with RapidArc therapy. The cohort included 6 males and 6 females, aged 42–76 years (median age: 57 years). Each patient had 3–5 brain metastases (45 metastases in total), with individual metastasis volumes ranging from 1.028 cm³ to 8.860 cm³ (average volume: 4.139 cm³).
1.2 Equipment and Systems
• RapidArc: Equipment: VitalBeam dual-photon linear accelerator (6 MV energy). Treatment planning system: Eclipse 15.1 (Varian, USA). MLC (Varian, USA): 60 leaf pairs; central 40 pairs had 5 mm width, outer 10 pairs per side had 10 mm width.
• Heavy Ion 2D Therapy: Equipment: Carbon ion therapy system HIMM-01-GS-WW-1 (Lanzhou Kejin Taiji New Technology Co., Ltd.). Treatment planning system: ciPlan (Shanghai Datu Medical Technology Co., Ltd. & Chinese Academy of Sciences Institute of Modern Physics). Accessories (Lanzhou Kejin Taiji New Technology Co., Ltd.): Ridge filter (30–120 mm), range shifter (min. precision 0.5 mm), MLC (50 independent leaf pairs, 8 mm width), PMMA compensator (longitudinal resolution 0.1 mm, transverse resolution 4.0 mm).
1.3 Treatment Plan Design
Patient imaging data collected using a large-bore CT simulator (Siemens, Germany) was used for planning. Both RapidArc and heavy ion 2D treatment plans were designed according to RTOG 0933 guidelines and clinical requirements. Planning target volumes (PTVs) were delineated, with prescription dose required to cover ≥95% of PTV (i.e., V₃₀ ≥95% for PTV), while minimizing dose to normal tissues.
1.3.1 Imaging Data Selection and Processing
• RapidArc: Supine position; immobilized using vacuum bags, thermoplastic masks, and CIVICO headrest (Germany); 1.5 mm slice thickness.
• Heavy Ion 2D: Fixed gantry angles required consideration of beam entry angles. Positioning: supine (same as RapidArc) or prone (using foam cushions and thermoplastic masks with CIVICO board); 1.5 mm slice thickness. For the 9 patients originally treated with RapidArc, heavy ion 2D planning required careful beam direction selection to avoid critical organs (e.g., eyes, optic nerves, brainstem).
1.3.2 RapidArc Plan Design
Designed using Eclipse TPS RapidArc (inverse planning). Optimization algorithm: anisotropic analytical algorithm (AAA); calculation grid: 2.5 mm. Isocenter placed at geometric center of multiple metastases. Four non-coplanar arcs used (Fig. 1A). Collimator angles set to 5°–15° (adjusted based on OAR avoidance and metastasis locations) ; X-jaw ≤15 cm. Max dose rate: 500 cGy/min 56. Prescription dose: 30 Gy to all metastases. For targets away from OARs: 98% isodose line covered 100% target volume; for targets near OARs: 95% isodose line covered 100% target volume.
1.3.3 Heavy Ion 2D Plan Design
Designed using ciPlan TPS 2D technique. Calculation grid: 2 mm. MLC (50 pairs) used for beam shaping in cross-section. PMMA compensator (100 mm) shaped dose fall-off at distal edge. Fixed gantry angles (0° & 90°) required "nearest-field" placement by adjusting couch angle and beam direction to avoid OARs. Single-field use minimized; 1–4 fields per patient, each field covering 1–3 metastases. Prescription dose: 30 Gy to all metastases. Plan design illustrated in Fig. 1B.
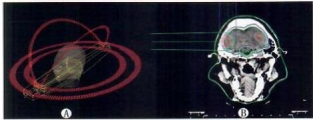
A: RapidArc treatment plan, B: Heavy ion 2D treatment plan
Fig.1 Distribution patterns of the two treatment plans
1.4 Dosimetric parameters
The dose conformity index (CI), dose homogeneity index (HI), and dose gradient index (GI) around the target were calculated for each target volume. A CI value closer to 1 indicates better conformity; an HI value closer to 0 indicates better homogeneity; a smaller GI value indicates a steeper dose gradient. The calculation formulas are as follows:
CI = (TV<sub>PV</sub>)² / (TV × PV),
HI = (D<sub>2%</sub> – D<sub>98%</sub>) / D<sub>mean</sub>,
GI = PV<sub>50%</sub> / PV.
Here, TV represents the target volume, PV is the volume enclosed by the prescription isodose line, TV<sub>PV</sub> is the target volume covered by the prescription dose, D<sub>2%</sub> is the dose received by 2% of the target volume, D<sub>98%</sub> is the dose received by 98% of the target volume, D<sub>mean</sub> is the mean dose to the target, and PV<sub>50%</sub> is the volume covered by the 50% prescription isodose line.
Dose exposure to organs at risk (OARs) and normal brain tissue was compared, including:
• Normal brain tissue: V<sub>5</sub>, V<sub>10</sub>, V<sub>15</sub>, V<sub>20</sub>, V<sub>25</sub>, V<sub>30</sub>
• Maximum dose to the left optic nerve (D<sub>OLmax</sub>)
• Maximum dose to the right optic nerve (D<sub>ORmax</sub>)
• Maximum dose to the optic chiasm (D<sub>OCmax</sub>)•Maximum dose to the brainstem (D<sub>BSmax</sub>)•Maximum dose to the left lens (D<sub>LLmax</sub>)
• Maximum dose to the right lens (D<sub>LRmax</sub>).
1.Results2.1 Comparison of target dosimetric parameters between the two treatment plansNo significant difference was observed in CI between the two plans (P > 0.05). However, significant differences were found in HI and GI (t = 6.199 for HI, t = -4.100 for GI; P < 0.05). See Table 1.
Tab.1 Comparison of CI, HI, and GI between two treatment plans (n=12)
| 指标 | 重离子二维 治 疗 计 划 | RapidArc 治疗计划 | t/z P |
| CIx±s) | 0.81±0.11 | 0.80±0.03 | 0.406 0.692 |
| HI(x±s) | 0.08±0.01 | 0.05±0.01 | 6.199 <0.001 |
| GI[M(P²5,P75)] | 3.08(2.66,3.38) | 9.06(8.50,9.65) | —4.100 <0.001 |
2.2 Comparison of irradiated normal brain tissue volumes between the two treatment plans
In the low-dose regions (V₅, V10, and V15), the irradiated volume of normal brain tissue in the two-dimensional heavy ion treatment plan was significantly smaller than that in the RapidArc treatment plan (t=4.285, 3.441, t=-2.309, P<0.05). In the high-dose region (V30), the irradiated volume of normal brain tissue in the two-dimensional heavy ion plan was significantly larger than that in the RapidArc treatment plan (t=-3.233, P<0.05). In the medium-dose regions (V20 and V25), there was no significant difference between the two treatment plans (P>0.05). See Table 2.
Tab.2 Comparison of irradiated volumes of normal brain tissue between the two treatment plans(V/cm³,n=12)
| 指标 | 重离子二维治疗计划 | RapidArc治疗计划 | 1/z | P |
| V;(r±s) | 226.79±118.76 | 655.51±325.60 | 一4,285 | 0.001 |
| Va(x±s) | 163.38±85.96 | 432.47±256.85 | —3.441 | 0.004 |
| Vis[M(Ps,Prs)] | 128.15(66.13,175.76) | 211.55(111.58,409.45) | —2.309 | 0.021 |
| V[M(Pa,Prs)] | 87.52(45.86,126.76) | 125.45(65.05,215.50) | -1.674 | 0.094 |
| Vz[M(Ps,P₇s)] | 54.72(27.84,77.52) | 57.00(28.85,90.25) | 一0.462 | 0.644 |
| V₃ Ω[M(P₂s,Ps)] | 13.56(7.41,20.63) | 3.40(1.96,8.93) | 一3.233 | 0.001 |
2.3 Comparison of the maximum radiation doses to organs at risk between the two treatment plans
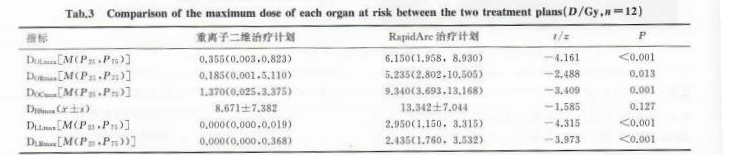
The maximum doses to the left lens (DOL<sub>max</sub>), right lens (DOR<sub>max</sub>), optic chiasm (DOC<sub>max</sub>), left optic nerve (DLL<sub>max</sub>), and right optic nerve (DLR<sub>max</sub>) in the two-dimensional heavy ion treatment plan were significantly lower than those in the RapidArc treatment plan (t= -4.315 to -2.488, P< 0.05). However, there was no significant difference in the maximum dose to the brainstem (DBS<sub>max</sub>) between the two treatment plans (P> 0.05). See Table 3.
Tab.3 Comparison of the maximum dose of each organ at risk between the two treatment plans(D/Gy,n=12)
2.4 Comparison of dose cloud maps and isodose line distributions between the two treatment plans
Both treatment plans met the clinical prescription requirements. The dose cloud maps and isodose line distributions showed that the prescription dose effectively covered the target volume without hot spots in both plans. In the two-dimensional heavy ion treatment plan, the low-dose region was significantly reduced, and the dose gradient was steep. The spinal cord and medulla oblongata received almost zero dose exposure (Figure 2A, 2B). In contrast, the RapidArc treatment plan exhibited a shallower dose gradient: the entire CT cross-section was largely covered by the 10% isodose line, with most areas covered by the 25% isodose line. The spinal cord and medulla oblongata were covered by the 50% isodose region (Figure 2C, 2D). Additionally, the two-dimensional heavy ion treatment plan generated a small volume of high-dose exposure at the intersection of fields, leading to an increased V30 outside the target volume (Figure 3).
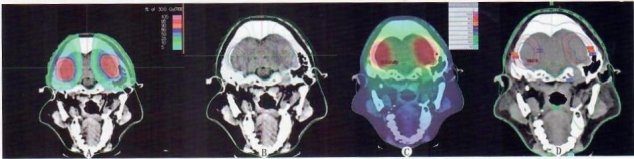
A and B are the dose cloud distribution and isodose line distribution of the two-dimensional heavy ion treatment plan, respectively; C and D are the dose cloud distribution and isodose line distribution of the RapidArc treatment plan, respectively.
Fig.2 Dose cloud and isodose map differences between the two treatment plans
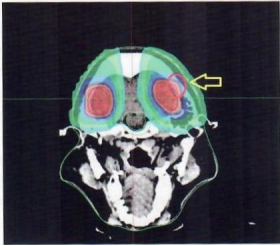
Fig.3 The 2D treatment plan for heavy ions generates a small volume of high-dose region at the intersection of the two fields
3. Discussion
The brain is the most complex organ in the human body. Radiation-induced neurological damage during radiotherapy for brain tumors is mostly irreversible, ranging from mild symptoms such as drowsiness, visual impairment, and memory decline to severe outcomes including decreased neural function and even radiation-induced brain necrosis [13-15]. Some studies suggest that even low doses of radiation to the brain may cause radiotherapy-related toxicity [16-17].
RapidArc is a form of volumetric modulated arc therapy (VMAT). Unlike static field intensity-modulated radiation therapy (IMRT), it enables the coordinated variation of the multi-leaf collimator (MLC), dose rate, and gantry during treatment, achieving stronger dose modulation capabilities. It represents a significant leap in IMRT technology and is one of the most advanced treatment techniques currently available on linear accelerators [18]. It allows treating multiple brain metastases at a single isocenter [19]. Studies have also reported that this technique offers high treatment efficiency and dosimetric coverage advantages in treating brain metastases [20].
With the continuous advancement of modern radiotherapy technology, a number of medical institutions worldwide have begun using proton and heavy ion therapy. The Institute of Modern Physics, Chinese Academy of Sciences, is one of the earliest research institutions in China to adopt heavy ion therapy for tumors. It has established heavy ion therapy centers domestically, utilizing a treatment planning system developed by the institute. Heavy ion therapy systems are among the most advanced radiotherapy devices of this century. Due to the unique physical properties of heavy ion beams, there are significant differences in physical and biological dose distributions between heavy ion therapy systems and conventional linear accelerator-based systems.
The results of this study show no significant difference in conformity index (CI) between the two treatment plans, meaning they provide the same prescription dose coverage. The homogeneity index (HI) of the two-dimensional heavy ion treatment plans was inferior to that of the mature RapidArc technology. This is because the two-dimensional heavy ion plans have limited modulation capability and use point-dose normalization for prescription dose calculation. To ensure sufficient target coverage of the prescription dose, dose homogeneity is compromised. The irradiated volume of normal brain tissue in the low-dose region (V5, V10, and V15) was significantly reduced in the two-dimensional heavy ion plans, and the gradient index (GI) was better. Owing to its unique physical characteristics, it better reduces the low-dose irradiation area and decreases the maximum dose to the lenses, optic nerves, and optic chiasm. In the medium-dose region (V20 and V25) of normal brain tissue, there was no significant difference between the two plans. However, in the high-dose region (V30), the two-dimensional heavy ion plan was inferior to the RapidArc plan. This is primarily because the two-dimensional heavy ion plan lacks modulation capability and can only use compensators for conformity at the target posterior edge. To achieve better target conformity, a small high-dose region is inevitable. This may be improved with future pencil beam scanning (3DSS) technology.
Currently, proton and heavy ion therapy are still in the development and promotion stage. The heavy ion equipment used in China is still being optimized, and some challenges remain unresolved [21-22]. For example: ① Due to the complexity of the equipment, the treatment head angles are fixed, making it difficult to provide non-coplanar beams; ② There are limited dose modulation methods. The current heavy ion therapy systems mainly use two-dimensional (2D) therapy, two-dimensional layer stacking (2DLS), and 3DSS. Among these, 3DSS can largely meet dose modulation requirements, and the treatment planning system uses the Monte Carlo method for more accurate dose calculation [23]. However, the fixed treatment head angles impose certain limitations on modulation capability in three-dimensional space. Studies have also shown that even with fixed treatment head angles, heavy ion therapy still offers advantages in dose distribution, such as reducing the maximum and mean doses to organs at risk, thereby effectively minimizing potential adverse reactions during radiotherapy [24].
In conclusion, as an advanced radiotherapy technique, heavy ion therapy has undeniable dosimetric advantages. The results of this study indicate that for treating brain metastasis patients, using only two-dimensional heavy ion technology without dose modulation cannot match some dosimetric metrics (e.g., CI, V30 outside the target) of the relatively mature RapidArc technology. However, due to its superior physical characteristics—such as reduced low-dose radiation areas, rapid dose fall-off at the target posterior edge, and minimal radiation exposure to organs at risk outside the beam entrance angles—it can effectively protect normal organs. This expands the applicability of radiotherapy for brain tumors, ultimately benefiting patients.
Conflicts of Interest: All authors disclose no relevant conflicts of interest.
References
[1]RÉGISJ,TAMURA M,GUILLOT C,et al.Radiosurgery with the world's first fully robotized leksell gamma knife per- fexion in clinical usea 200-patient prospective,randomized, controlled comparison with the gamma knife 4c[J].Neurosur- gery,2009,64(2):346-356.
[2]葛琴,钱霞,吴建亭,等.不同剂量分割模式放疗对局部晚期 NSCLC患者血清EGFR、TGF-a的影响[J].现代肿瘤医学,2019,27(2):229-232.
GE Q,QIAN X,WUJT,et al.Effect of different dose frac- tionated radiotherapy on serum EGFR and TGF-a in patients with locally advanced non-small cell lung cancer[J].J Mod Oncol,2019,27(2):229-232.
[3]SOFFIETTIR,RUDA R,MUTANI R.Management of brain metastases[J].J Neurol,2002,249(10):1357-1369.
[4]LINDQUIST C,PADDICK I.The leksell gamma knife perfe- xion and comparisons with its predecessors[J].Neurosurge ry,2008,62(Suppl2):721-732.
[5]SHEEHANJP,SUN MH,KONDZIOLKA D,et al.Radio- surgery for non-small cell lung carcinoma metastatic to the brain:Long-term outcomes and prognostic factors influencing patient survival time and local tumor control[J].J Neurosurg,2002,97(6):1276-1281.
[6]李勇,潘绵顺,邱书珺,等.非小细胞肺癌脑转移瘤的立体定向放射治疗[J].中华神经外科杂志,2014,30(7):711-714.
LI Y,PAN MS,QIU SJ,et al.Therapeutic effect of ste- reotactic radiotherapy for brain metastases of non-small cell lung cancer[J].Chinese Journal of Neurosurgery,2014,30(7):711-714.
[7]张平,邓官华,戴鹏,等.RapidArc与HybridArc技术在大体积脑转移瘤立体定向放射外科中的剂量学比较[J].中国医学物理学杂志,2019,36(8):887-891.
ZHANG P,DENG GH,DAI P,et al.Stereotactic radio- surgery for large brain metastases:A dosimetric comparison of volumetric modulated are therapy generated with RapidArc versus HybridArc[J].Chin JMed Phys,2019,36(8):887-891.
[8]贾蓉,苏锋涛,胡步荣.重离子的辐射生物效应及其在生命科学中的应用[J].生物技术通报,2018,34(1):67-78.
JIA R,SU FT,HU BR.The biological effects induced by heavy ion radiation and its application in life science[J].Biotechnol Bull,2018,34(1):67-78.
[9]张平,戴鹏,罗龙辉,等.准直器角度对颅内两个脑转移瘤容积旋转调强计划的影响[J].中国医学物理学杂志,2018,35(12):1399-1403.
ZHANGP,DAI P,LUOLH,et al.Effects of collimator angle on volumetric modulated are therapy plans for two brain metastases[J].Chin J Med Phys,2018,35(12):1399-1403.
[10]HSU F,CAROLAN H,NICHOL A,et al.Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases:A feasibility study using volumetric modulated arc therapy[J].Int J Radiat Oncol Biol Phys,2010,76(5):1480-1485.
[11]THOMASEM,POPPLE RA,WU XG,et al.Comparison of plan quality and delivery time between volumetric arc thera- py(RapidArc)and Gamma Knife radiosurgery for multiple cranial metastases[J].Neurosurgery,2014,75(4):409-417
[12]MORRISON J,HOOD R,YIN FF,et al.Is a single iso- center sufficient for volumetric modulated arc therapy radio- surgery when multiple itracranial metastases are spatially dis- persed?[J].Med Dosim,2016,41(4):285-289.
[13]ATTIA A,RAPP SR,CASE LD,et al.PhaseⅡstudy of Ginkgo biloba in irradiated brain tumor patients:Effect on cognitive function,quality of life,and mood[J].J Neurooncol.2012,109(2):357-363.
[14]POWELLC,SCHICK U,MORDEN JP,et al.Fatigue after chemoradiotherapy for nasopharyngeal cancer and its rela- tionship to radiation dose distribution in the brain[J].Radio- ther Oncol,2014,110(3):416-421.
[15]汪步海,李颖,刘丽琴,等.应用海马保护技术预防全脑放疗患者认知功能障碍研究[J].中华肿瘤防治杂志,2015,22(18):1470-1474.
WANG BH,LI Y,LIU LQ,et al.Application of hippocam- pal protection technology to prevent cognitive dysfunction in patients with whole brain radiation therapy[J].Chin J Cancer Prev Treat,2015,22(18):1470-1474.
[16]吕明惠,苏少华,郑婉君,等.放射性脑损伤研究进展[J].亚太传统医药,2017,13(17):79-82.
LYU MH,SU SH,ZHENG WJ,et al.Advances in re- search of radiation brain injury[J].Asia Pac Tradit Med,2017,13(17):79-82.
[17]FLICKINGERJC,KONDZIOLKA D,LUNSFORD LD,et al.Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation pa- tients.Arteriovenous Malformation Radiosurgery Study Group[J].Int J Radiat Oncol Biol Phys,2000,46(5):1143-1148.
[18]徐英杰,肖建平,马攀,等.容积调强弧形治疗用于单发脑转移瘤的剂量学研究[J].中华放射肿瘤学杂志,2015,24(3):306-309.
XUYJ,XIAOJP,MA P,et al.Dosimetry study of volu- metric-modulated arc therapy for single brain metastasis[J].Chin J Radiat Oncol,2015,24(3):306-309.
[19]ROBERGE D,RUO R,SOUHAMI L.Killing two birds with one stone:A dosimetric study of dual target radiosurgery using a single isocenter[J].Technol Cancer Res Treat,2006,5(6):613-617.
初审:张莉红 复审:张洁
