Can't Afford 1.2 Million Yuan CAR-T? Clinical Trials Offer New Hope!
Can't Afford 1.2 Million Yuan CAR-T? Clinical Trials Offer New Hope!
--Clinical Trial Patient Recruitment Announcement: CAR-T Therapy for Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma (B-NHL), B-Cell Acute Lymphoblastic Leukemia (B-ALL), and Multiple Myeloma (MM)
Project Background
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel precision-targeted cellular treatment approach. This innovative therapy involves collecting a patient's own T cells, genetically modifying them to specifically recognize and attack diseased cells, and then reinfusing the engineered cells back into the patient. Currently, CAR-T therapy has demonstrated excellent clinical safety and efficacy in the treatment of hematologic malignancies.
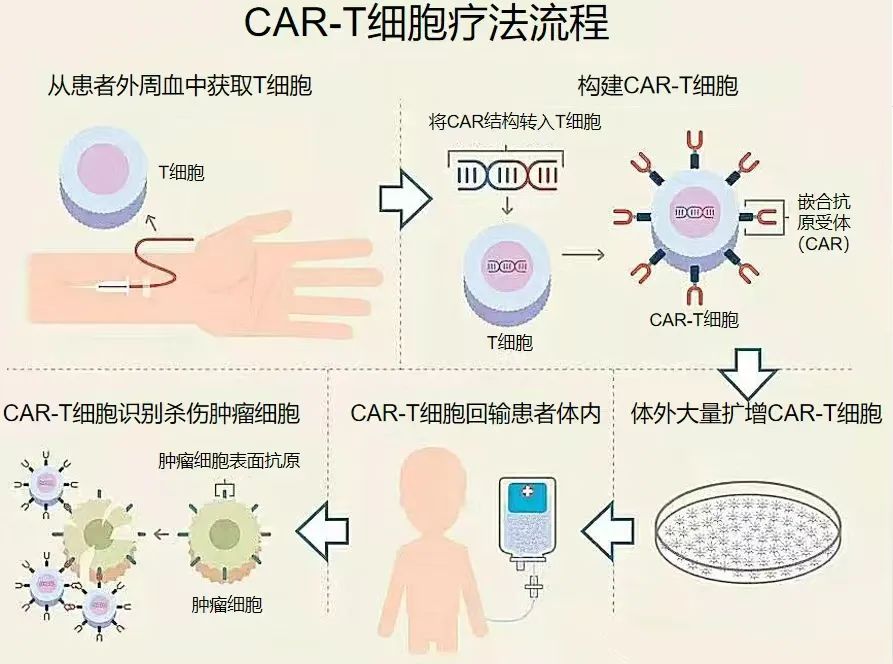
Approved by the Ethics Committee of Gansu Wuwei Cancer Hospital, two CAR-T cell therapy clinical trials are currently underway: a clinical study of humanized CD19-targeted CAR-T cells for relapsed/refractory B-cell hematologic malignancies (including B-cell non-Hodgkin lymphoma and B-cell acute lymphoblastic leukemia), and a humanized BCMA-targeted CAR-T cell therapy for relapsed/refractory multiple myeloma (MM), bringing new hope to more patients.
Department Background
This project is led by Director Wang Cong of the Hematology Department. Director Wang Cong holds multiple prestigious academic appointments, including:Youth Committee Member of the 1st Youth Working Group, Hematological Disease Rehabilitation Committee, Chinese Association of Rehabilitation Medicine,Committee Member of the Blood Precision Diagnosis and Treatment Committee, Chinese Research Hospital Association,Committee Member of the Lymphoma Professional Committee, China Health Technology Promotion Association
Standing Committee Member of the 1st Lymphoma Committee, Gansu Provincial Alliance of China Cancer Prevention and Treatment Union
Standing Committee Member of the 1st Blood Disease Translational Medicine Committee, Gansu Anti-Cancer Association
Expert at the Gansu Provincial Hematology Quality Control Center
Standing Committee Member of the Hematology Physician Branch, Gansu Medical Doctor Association
Standing Committee Member of the 1st Hematology Committee, Gansu Association of Integrated Traditional Chinese and Western Medicine
Committee Member of the Cell Therapy Committee, Shaanxi Research Hospital Association
Committee Member of the 2nd Lymphoma Committee, Gansu Anti-Cancer Association
Committee Member of the Hematology Committee, Integrated Medicine Physician Branch, Gansu Medical Doctor Associatio
Vice Chairperson of the Wuwei Hematology Committee, Wuwei Medical Association
Vice Chairperson of the 1st Hematology Committee, Wuwei Association of Integrated Traditional Chinese and Western Medicine
Expert at the Wuwei Hematology Quality Control Center
Outstanding Expert in the "People's Good Doctor" (Lymphoma Field) Program
With extensive clinical and basic research experience in hematological diseases, Director Wang Cong specializes in the diagnosis and treatment of acute/chronic leukemia, lymphoma, multiple myeloma, myelodysplastic syndromes, various anemias, and thrombocytopenia. In clinical trials, Director Wang Cong has led and participated in numerous clinical research projects.

The Department of Hematology at Gansu Wuwei Medical Science Academy consists of four subspecialties: a hematology ward, hematopoietic stem cell transplantation unit, hematology laboratory, and Wuwei Hematology Research Institute, with 50 open beds. In 2019, it was selected as one of China's first member units of the Pediatric Hematologic Diseases and Malignancies Diagnosis and Treatment Collaborative Group. In May 2021, it was recognized as a key municipal discipline in Wuwei. In 2023, it joined the Northwest Hematology Alliance, became a branch center of the Gansu Provincial Clinical Research Center for Leukemia, and was included as a member unit of the Northwest Four-Province CLL Working Group. In February 2024, it was designated as a cultivation center for the ITP Comprehensive Management Quality Improvement Program by the Thrombosis and Hemostasis Group of the Hematology Branch of the Chinese Medical Association. In August 2024, it was honored as an Outstanding Physician Team in the city. The department currently has 1 associate chief physician, 2 attending physicians, 1 laboratory chief technician, 4 resident physicians, and 11 nursing staff.
The department specializes in hematopoietic stem cell transplantation (including autologous and allogeneic transplantation), blood cell separation technology, bone marrow aspiration and biopsy, lumbar puncture with intrathecal injection, PICC catheter placement and maintenance, port maintenance, and emergency care for critically ill hematology patients. Its diagnostic and therapeutic capabilities for leukemia, lymphoma, multiple myeloma, aplastic anemia, and myelodysplastic syndromes have reached leading levels in the Hexi region. The department has established significant specialty advantages in the diagnosis, treatment, and efficacy evaluation of hematologic diseases.
Adhering to the integrated development of clinical hematology and laboratory research, the department utilizes flow cytometry, coagulation factor analyzers, erythrocyte lifespan analyzers, agarose gel electrophoresis, Olympus optical microscopy, and immunohistochemistry for the diagnosis and classification of various hematologic disorders. It was the first in the Hexi region to introduce advanced diagnostic technologies in hematologic molecular biology, cell biology, immunology, cytogenetics, and morphology, enabling standardized diagnosis and efficacy assessment of hematologic diseases according to international and domestic guidelines.
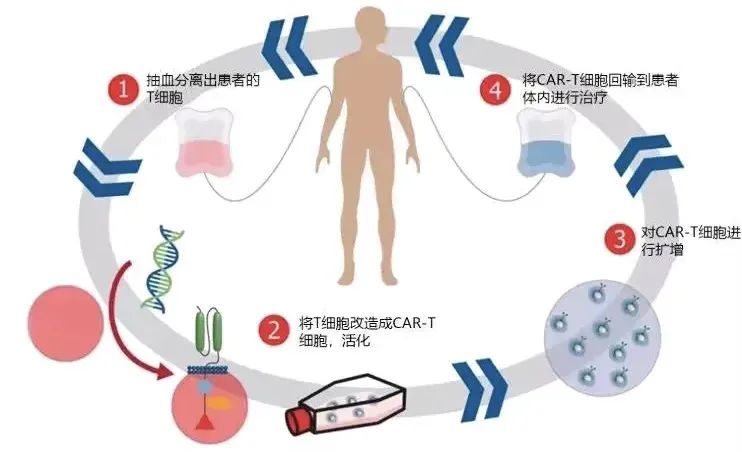
Introduction to the Clinical Drug
Investigational Drug: CAR-T Cell Therapy Injection
Administration Method: Intravenous infusion
Mechanism of Action:
T cells, as one of the most important immune cells in the human body, serve as elite forces in eliminating tumor cells and infected cells. CAR-T therapy equips these "troops" with specialized targeting heads called "CARs," enabling them to precisely identify surface markers on tumor cells. This transforms their recognition mechanism from complex "friend-or-foe" identification to immediate detection of malignant tumor cells upon contact, allowing direct attacks.
Such rapid identification and clearance prevent tumors from employing various evasion mechanisms to conceal their identity and deceive the immune system. Consequently, CAR-T cell therapy has demonstrated remarkable efficacy, establishing itself as one of the most promising immunotherapies available.

Key Inclusion Criteria
Candidates must be able to communicate effectively with investigators and provide written informed consent.
1 Age: 18 to 70 years (inclusive), any gender.
2 Aggressive non-Hodgkin lymphoma (NHL) confirmed by cytology/histology per Lymphoma Diagnosis and Treatment Guidelines (2018 Edition), including diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma, etc., with CD19-positive expression.
3.Relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) with CD19-positive expression.
4.Active multiple myeloma (MM) diagnosed per Chinese Guidelines for Diagnosis and Treatment of Multiple Myeloma (2017 Revision), with BCMA positivity (≥5% abnormal plasma cells by flow cytometry).
5.For B-cell lymphoma: Must meet relapse/refractory criteria:
Relapse within 12 months after first-line therapy containing rituximab (or other CD20-targeted drugs) and anthracyclines;
6.Primary refractory to first-line therapy;
Relapse after ≥2 prior systemic therapies (including autologous hematopoietic stem cell transplantation) containing rituximab/anthracyclines;
Or relapsed patients not fully meeting above criteria but refusing chemotherapy and strongly opting for CAR-T therapy.
≥1 measurable lesion: nodal lesion long axis >1.5 cm or extranodal lesion long axis >1.0 cm.
Relapsed/refractory B-ALL after first-line therapy
7.For MM refractory/relapsed definition:
a) Never achieved minimal response or better to any prior therapy.
b) No response to salvage therapy or progression within 60 days post-last treatment.
c) Clinical relapse (per 2020 Chinese MM Guidelines): ≥1 of:
i. New bone lesions (clonal plasma cells ≥10%) or soft tissue plasmacytomas (excluding osteoporotic fractures);
ii. ≥50% increase in measurable lesions (SPD increase ≥1 cm);
iii. Hypercalcemia (>2.75 mmol/L);
iv. Hgb drop ≥20 g/L (unrelated to therapy/non-MM factors);
v. Serum creatinine ≥177 μmol/L (MM-related);
vi. Serum M protein-related hyperviscosity.
d) Post-CR relapse: ≥1 of:
i. Reappearance of serum/urine M protein by immunofixation;
ii. Bone marrow plasma cells ≥5%;
iii. New clinical progression (e.g., plasmacytoma/lytic lesions/hypercalcemia).
e) Disease progression (per 2020 Chinese MM Guidelines): ≥1 of:
i. Serum M protein increase ≥25% (absolute ≥5 g/L; if baseline ≥50 g/L, ≥10 g/L suffices);
ii. Urine M protein increase ≥25% (absolute ≥200 mg/24h);
iii. If no detectable M protein, serum FLC difference increase ≥25% (absolute >100 mg/L);
iv. Bone marrow plasma cells increase ≥25% (absolute ≥10%);
v. ≥25% increase in existing bone/soft tissue lesions or new lesions;
vi. Hypercalcemia (corrected calcium >2.8 mmol/L);
vii. Two consecutive assessments required pre-treatment
9.Blood counts: Neutrophils ≥1.0×10⁹/L; hemoglobin ≥70 g/L; platelets ≥50×10⁹/L.
10.Coagulation: Fibrinogen ≥1.0 g/L; APTT ≤ULN+10 s; PT ≤ULN+3 s
11.Liver/kidney function:
Total bilirubin ≤1.5×ULN (excluding Gilbert’s/hemolysis);
ALT/AST ≤3×ULN;
Serum creatinine ≤2×ULN.
12.Exceptions allowed if abnormalities are tumor-related.
Cardiac: LVEF ≥45% by ECHO/MUGA (post-treatment correction permitted).
Pulmonary: Dyspnea ≤CTCAE grade 1; room-air SpO₂ ≥92%.
ECOG performance status 0–2
Expected survival >3 months.
dequate organ function at screening.
Women of childbearing potential: Negative pregnancy test at screening/pre-chemotherapy; agreement to use highly effective contraception for 1 year post-treatment.
Men with fertile partners: Must use highly effective contraception for 1 year post-treatment; sperm donation prohibited for 1 year.

Key Exclusion Criteria
1. History of hypersensitivity to human albumin or DMSO.
2. Active HBV infection (HBV-DNA positive), HCV infection (HCV-RNA positive), or HIV infection.
3. End-organ damage due to autoimmune diseases (e.g., Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus) within the past 2 years, or requiring systemic immunosuppressants/disease-modifying agents.
4. Unstable angina, myocardial infarction, coronary angioplasty, or significant cardiac disease within the past year.
5. Uncontrolled psychiatric disorders.
6. Concurrent life-threatening organ failure.
7. Participation in other clinical trials within 4 weeks prior to enrollment.
8. Live vaccination within 4 weeks before enrollment.
9. Use of prohibited medications:
- Corticosteroids: Therapeutic doses (>20 mg/day prednisone or equivalent) within 7 days before leukapheresis or 48 hours before CAR-T infusion. *Physiologic replacement, topical, or inhaled steroids are permitted.*
- Chemotherapy: Salvage therapy (including TKIs) within 1 week before leukapheresis.
- Donor lymphocyte infusion (DLI): Within 4 weeks before leukapheresis.
- GVHD treatment: Systemic anti-GVHD therapy within 3 months before CAR-T infusion.
- Alemtuzumab: Use within 6 months before leukapheresis; cladribine or clofarabine within 3 months.
- Checkpoint inhibitors/agonists: Use prior to enrollment (unless >3 half-lives have elapsed).
10. Prior pulmonary injury or hemorrhagic cystitis related to cyclophosphamide.
11. Pregnancy, planned pregnancy during the trial, or lactation.
12. Inability to comply with study requirements (including follow-up) per investigator assessment.

For further information about this study, please contact us via the following:
Address:
Department of Hematology, Gansu Wuwei Cancer Hospital
(Heavy Ion Treatment Center, Building 1, 3rd Floor / Main Hospital Outpatient Room 203)
Contact Physicians:
- Director Wang Cong
- Dr. Zhang Yuxia
- Dr. Han Xueping
- Dr. Zhao Haitao
Phone Numbers:
- Director Wang Cong: +86 138 9351 0690
- Dr. Zhang: +86 182 9355 4683
- Dr. Han: +86 151 9354 0791
