Clinical Study on Heavy Ion Therapy with Zero Self-Payment for High-Grade Glioma Fully Launched
Clinical Study on Heavy Ion Therapy with Zero Self-Payment for High-Grade Glioma Fully Launched
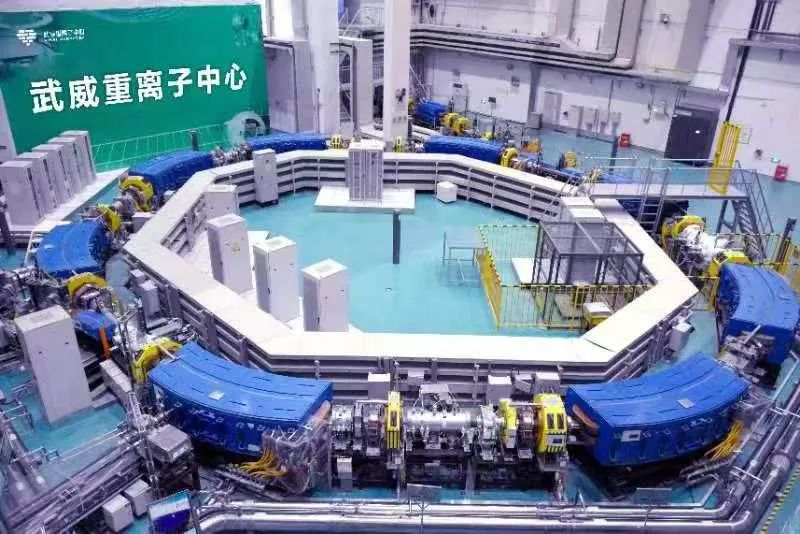
Gansu Wuwei Cancer Hospital – China's pioneer in clinical application of domestic heavy ion systems and the world's only facility operating dual heavy ion systems – has successfully treated over 2,000 patients across 50+ tumor types (including lung, pancreatic, and liver cancers) through five years of clinical practice. Clinical efficacy and survival rates meet international advanced standards.
The hospital has achieved "eight global and domestic firsts":
1. Only facility worldwide operating dual heavy ion therapy systems
2. Global leader in single-fraction treatments completed within 24 hours
3. Pioneer of ventilator-controlled precision heavy ion therapy
4. First to implement bladder cancer treatment via precise volume management
5. First to perform organ-tumor spacer-based single-session therapy
6. China's first developer of 360° rotatable ion therapy chair
7. First institution completing multiple cardiac tumor treatments with heavy ions
8. First to integrate heavy ion therapy with comprehensive lifelong health management
Building on these achievements, we now launch the Phase II Clinical Study: Carbon Ion Combined with Photon Radiotherapy for High-Grade Glioma (HGG). This initiative establishes China's clinical standards for glioma treatment using heavy ion technology aligned with global benchmarks to optimize patient outcomes.




Phase II Clinical Study: Carbon Ion Combined with Photon Radiotherapy for High-Grade Glioma
Study Objective
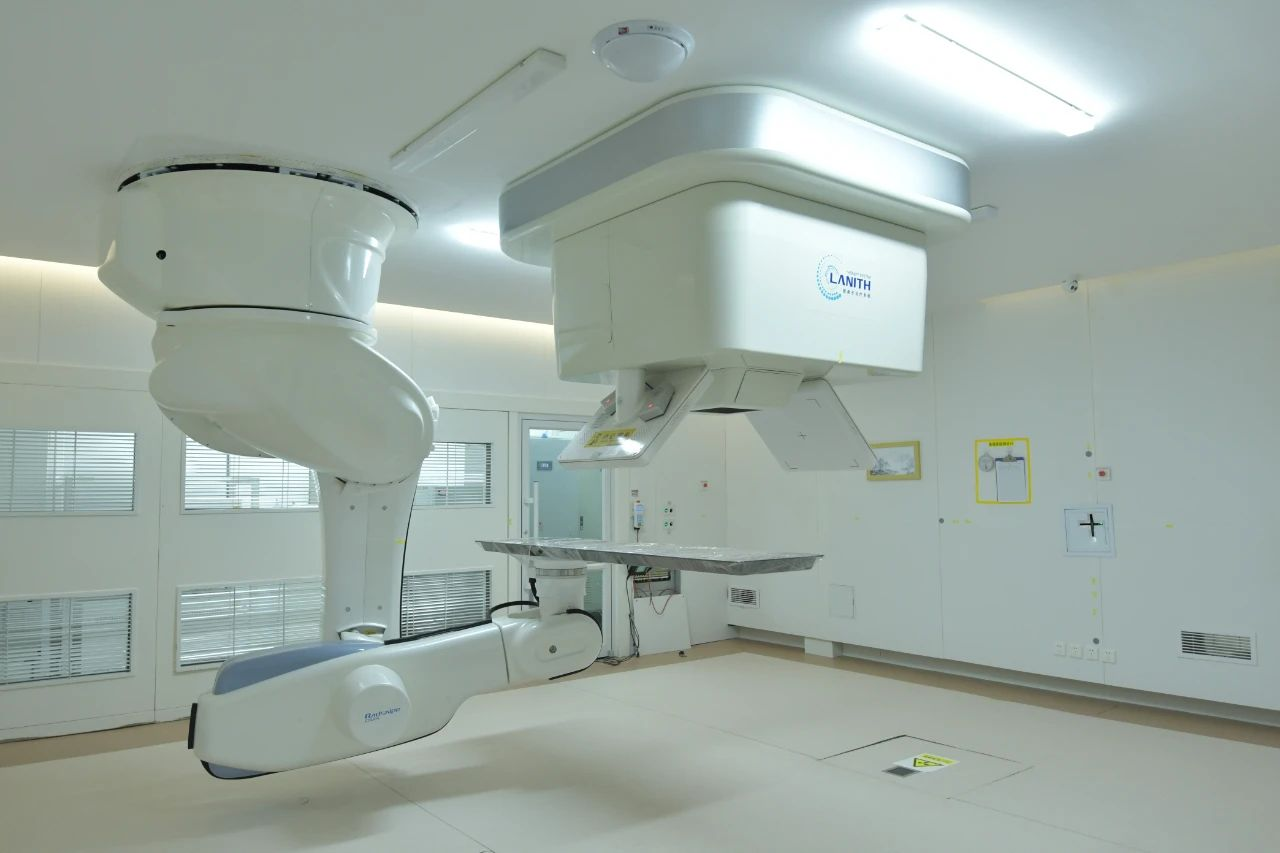
Led by Principal Investigators Dr. Li Xiaojun (Lanzhou Heavy Ion Center), Dr. Wang Junjie (National Chair of Radiation Oncology), and Dr. Hu Tingchao (Wuwei Heavy Ion Center), this single-center, single-arm prospective Phase II trial (Jan 2025-Dec 2027) evaluates a novel treatment protocol for HGG. Based on Japan's NIRS regimen showing 26-month median survival for glioblastoma (surpassing conventional radiotherapy), we will assess safety and efficacy of carbon ion-photon combination therapy. The study aims to leverage carbon ions' physical and biological advantages to enhance tumor control and long-term survival while reducing radiation-induced brain damage. 23 eligible patients will receive photon radiotherapy (IMRT/VMAT costs covered by medical insurance) plus carbon ion therapy (¥156,000/patient covered by hospital). Neuro-oncological 11C-MET PET scans offered at discounted rate (¥5,946/session vs. standard ¥8,919).
Inclusion Criteria
- Aged 14-80 years
- HGG diagnosis per WHO CNS5 (2021) criteria:
- Single intracranial lesion or dual lesions coverable by one radiation field
- No prior radiotherapy within 4 weeks; surgical wounds healed
- MRI/contrast CT compatible (no metal artifacts in CTV)
- No active secondary malignancies (except cured skin/cervical carcinoma in situ)
- Normal organ function:
- ECOG 0-2; NYHA Class I cardiac function
- Adequate major organ reserve
- Expected survival ≥6 months
- Signed informed consent
Final eligibility determined by investigators
Patient Benefits
1. Hospital-funded carbon ion therapy
2. Personalized MDT-developed treatment plan
3. Close follow-up monitoring and subsequent care planning
Contact Information
Lanzhou Heavy Ion Center
Dr. Li Xiaojun: +86 131 5016 0200
Wuwei Heavy Ion Center
Dr. Hu Tingchao: +86 138 9354 0404