Clinical Study on Zero Out-of-Pocket Heavy Ion Therapy for Esophageal Cancer Fully Launched
Clinical Study on Zero Out-of-Pocket Heavy Ion Therapy for Esophageal Cancer Fully Launched
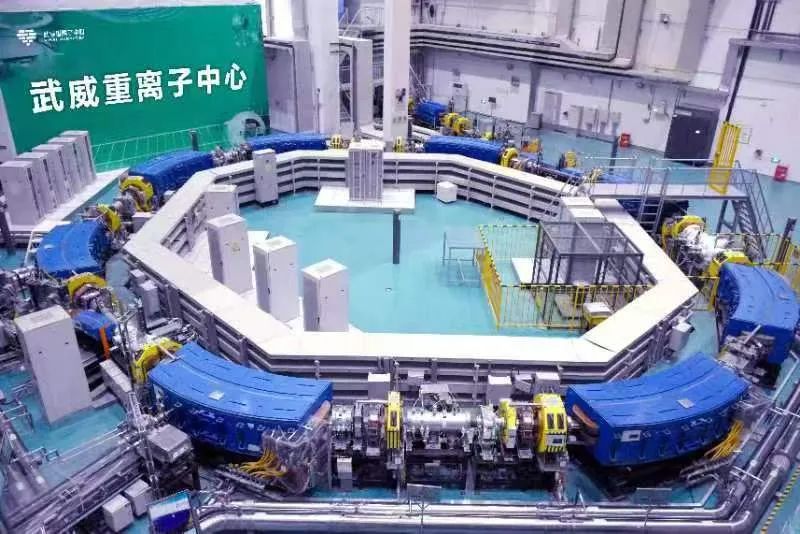
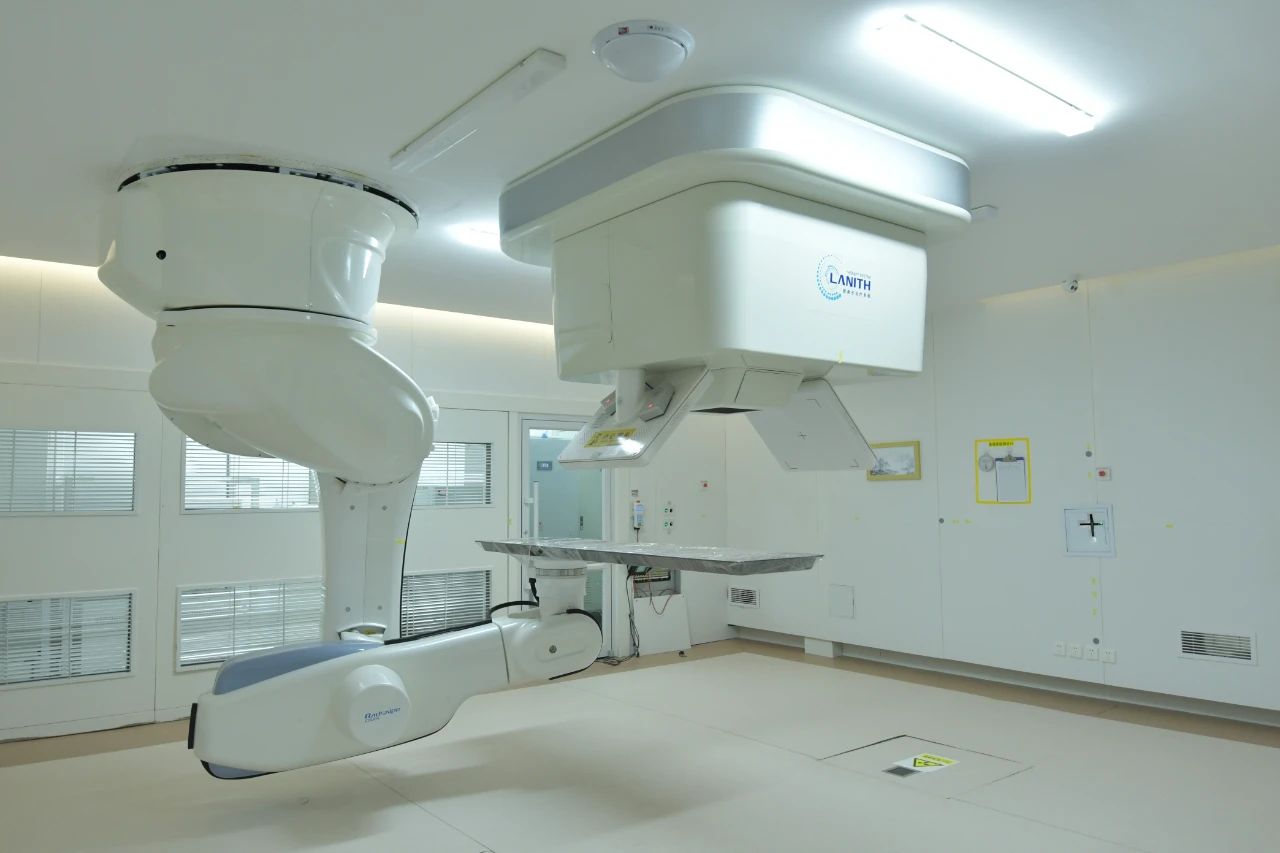
As the first hospital in China to clinically apply a domestic heavy ion system and the only hospital globally operating two heavy ion systems, Gansu Wuwei Cancer Hospital has accumulated five years of clinical experience, treating over 2,000 patients across more than 50 conditions, including lung cancer, pancreatic cancer, and liver cancer. Clinical efficacy and patient survival rates have reached internationally advanced levels. The hospital has achieved "eight world and domestic firsts": the only hospital globally operating two heavy ion therapy systems; the hospital with the highest number of single-fraction treatments completed within one day; the first globally to develop precision heavy ion therapy under ventilator control; the first globally to implement bladder cancer treatment with precise bladder volume management; the first globally to perform single-session heavy ion therapy using surgically placed spacers between organs and tumors; the first in China to develop a 360° rotatable and adjustable ion therapy chair; the first globally to complete multiple cases of cardiac tumor treatment with heavy ions; and the first globally to integrate heavy ion therapy with comprehensive, lifelong physical and mental health management.
To further advance the high-quality development of heavy ion therapy in China, the hospital will deepen clinical research and drive technological upgrades based on prior achievements. The "Phase II Clinical Study on Photon Combined with Carbon Ion Radiotherapy for Locally Advanced Esophageal Cancer" has now been launched, aiming to establish China’s clinical standards for heavy ion therapy in esophageal cancer, aligned with international benchmarks, to pursue better patient outcomes.




Phase II Clinical Study on Photon Combined with Carbon Ion Radiotherapy for Locally Advanced Esophageal Cancer
Study Objective
Gansu Wuwei Cancer Hospital plans to conduct this clinical trial from May 2025 to May 2029. The study aims to explore new treatment options for patients with esophageal cancer who are not candidates for surgery. A total of 32 participants will be enrolled. Successfully enrolled patients will undergo treatment for 6-7 weeks. Participation in this study exempts patients from heavy ion radiotherapy-related costs.
Inclusion Criteria
1. Age 18–70 years (inclusive);
2. Meets AJCC 8th edition staging criteria: cT1-4N0-3M0 (excluding cT1-2N0M0);
3. No prior radical radiotherapy or surgery for esophageal cancer;
4. Normal liver and kidney function;
5. Normal bone marrow function;
6. Cardiopulmonary function sufficient to tolerate radiotherapy.
The above criteria are primary screening standards; final eligibility will be determined by investigators based on the trial protocol.
Exclusion Criteria
1. Esophageal malignancy without pathological confirmation;
2. Organs at risk cannot meet dose constraint requirements;
3. High risk of esophageal perforation;
4. Presence of abdominal lymph node metastasis;
5. Implanted metal devices that may affect dose distribution or pose radiotherapy safety risks (e.g., pacemakers);
6. Absolute contraindications to radiotherapy.
Note: Final enrollment eligibility will be assessed by a multidisciplinary team (MDT).
Patient Benefits
1. The hospital will provide research funding to cover heavy ion therapy costs.
2. A multidisciplinary expert team (MDT) will develop a personalized treatment plan.
3. Close follow-up monitoring post-treatment, including provision of subsequent treatment plans.
Contact Information
Wuwei Heavy Ion Center, Gansu Wuwei Cancer Hospital
Chen Dongji: +86 138 9351 9363
Lanzhou Heavy Ion Center, Gansu Wuwei Cancer Hospital
Li Xiaojun: +86 131 5016 0200